Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Use the following information to determine the enthalpy for the reaction shown below. CS2(l) 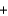 3O2(g)
3O2(g)  CO2(g)
CO2(g) 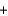 2SO2(g) H ?
C(graphite)
2SO2(g) H ?
C(graphite) 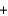 O2(g)
O2(g)  CO2(g) H 393.5 kJ
S(s)
CO2(g) H 393.5 kJ
S(s) 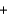 O2(g)
O2(g)  SO2(g) H 296.8 kJ
C(graphite) 2S(s)
SO2(g) H 296.8 kJ
C(graphite) 2S(s)  CS2(l) H 87.9 kJ
CS2(l) H 87.9 kJ
(Multiple Choice)
4.9/5  (28)
(28)
For which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation?
(Multiple Choice)
4.8/5  (42)
(42)
Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form, and its energy of combustion is known very accurately (26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 8.199C when 2.500 g of benzoic acid was used.
(Short Answer)
4.9/5  (34)
(34)
Describe the difference between the change in the internal energy (E) of a system and the change in enthalpy (H). Begin your description with the definition of enthalpy change.
(Essay)
4.7/5  (29)
(29)
Given the following thermochemical equation detailing the combustion of methane CH4(g) 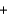 2O2(g)
2O2(g)  CO2(g)
CO2(g) 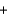 2H2O(g) Hrxn 802kJ/mol CH4
Determine the amount of energy released when 25.0 g of methane undergoes combustion.
2H2O(g) Hrxn 802kJ/mol CH4
Determine the amount of energy released when 25.0 g of methane undergoes combustion.
(Multiple Choice)
4.8/5  (35)
(35)
The integrated circuits in your cell phone and computer are made from the semiconductor silicon. The silicon is obtained from a really inexpensive starting material, sand, which is primarily SiO2. One step in the purification of silicon is to separate it from solid impurities by forming the gas silicon tetrachloride. Given the following reactions, what is the overall enthalpy change in converting 1.00 mol of silicon dioxide into pure silicon? ReactionH (kJ)
SiO2(s) 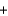 2C(s)
2C(s)  Si(impure s)
Si(impure s) 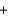 2CO(g)690
Si(impure s)
2CO(g)690
Si(impure s) 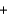 2Cl2(g)
2Cl2(g)  SiCl4(g)657
SiCl4(g)
SiCl4(g)657
SiCl4(g) 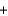 2Mg(s)
2Mg(s)  2MgCl2(s)
2MgCl2(s) 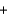 Si(s)625
Si(s)625
(Multiple Choice)
4.9/5  (34)
(34)
Explain what is meant by the term state function, and give an example of something that is a state function and something that is not a state function.
(Essay)
4.8/5  (43)
(43)
Isooctane is a good model compound for gasoline. When 1.14 g of isooctane (114 g/mol) reacts with excess oxygen in a constant pressure calorimeter, the temperature of the calorimeter increases by 10.0oC. The heat capacity of the calorimeter is 5.46 kJ/oC. Determine the molar enthalpy of combustion of isooctane.
(Multiple Choice)
4.8/5  (51)
(51)
Ethanol (CH3CH2OH) has been suggested as an alternative fuel source. Ethanol's enthalpy of combustion is Hcomb 1,368 kJ/mol, and its density is 0.789 g/mL. What is the fuel value of ethanol (kJ/g)?
(Multiple Choice)
4.8/5  (45)
(45)
According to Coulomb's law, which ionic compound A-D has the smallest electrostatic potential energy (i.e., closest to zero)? The size of the anion increases in the order F < Cl < Br < I.
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following objects will cool the fastest from the same initial temperature, assuming you had equal masses of each?
(Multiple Choice)
4.9/5  (29)
(29)
In an experiment, 5.0 g of ice at 12 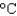 is converted into steam with a temperature of 120
is converted into steam with a temperature of 120 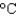 . How much energy is required for this process?
. How much energy is required for this process?  vap 2,260 J/g;
vap 2,260 J/g;  Hfus 334 J/g; cs(ice) 2.06 J/(g
Hfus 334 J/g; cs(ice) 2.06 J/(g  ); cs(water) 4.18 J/(g
); cs(water) 4.18 J/(g 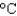 ); cs(steam) 1.99 J/(g
); cs(steam) 1.99 J/(g 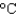 ))
))
(Multiple Choice)
4.8/5  (33)
(33)
Energy that an object has by virtue of its position is called ________
(Multiple Choice)
4.9/5  (41)
(41)
What will be the final temperature of a 10.0 g piece of iron (CP 25.09 J/(mol · 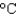 )) initially at 25C, if it is supplied with 9.5 J from a stove?
)) initially at 25C, if it is supplied with 9.5 J from a stove?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following bar charts shows the correct internal energy changes that occur when a propane grill is used to cook a steak? (Consider the propane combustion reaction to be the system. Consider the grill, steak, and everything else to be the surroundings.)
(Multiple Choice)
4.9/5  (30)
(30)
Assuming that the distance between ions remains constant in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i.e., largest in magnitude)?
(Multiple Choice)
4.8/5  (44)
(44)
A cooling curve for some substance is shown below. Which of the line segments (I-V) represents cooling of the gas? 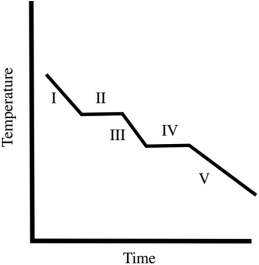
(Multiple Choice)
4.8/5  (38)
(38)
The diagram below shows three ion pairs: (a) a doubly charged anion and cation, (b) a singly charged anion and cation, and (c) two doubly charged anions.
(I) Label each pair to identify the electrostatic interaction as attractive or as repulsive.
(II) Which pair has the largest electrostatic interaction energy that is positive?
(III) Which pair has the largest electrostatic interaction energy that is negative?
(IV) Which pair has the smallest electrostatic interaction energy? 

 (a) (b) (c)
(a) (b) (c)
(Essay)
4.9/5  (38)
(38)
A cooling curve for some substance is shown below. Which of the line segments (I-V) represents the liquid-to-solid transition? 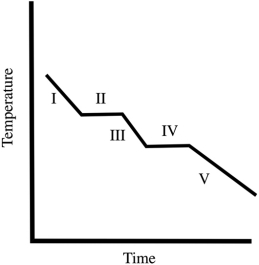
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)