Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
When ammonium nitrate (NH4NO3)(s) is used in explosives, it decomposes to N2(g), O2(g), and H2O(g). Write a balanced reaction equation for the decomposition of ammonium nitrate, and determine the standard enthalpy of reaction using standard enthalpies of formation:
solid ammonium nitrate 366 kJ/mol and water vapor 242 kJ/mol.
(Essay)
5.0/5  (43)
(43)
Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction.
2N2H4(g)  2NO2(g)
2NO2(g)  3N2(g)
3N2(g)  4H2O(g) Hrxn ? kJ
SubstanceH
4H2O(g) Hrxn ? kJ
SubstanceH  in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
(Short Answer)
4.8/5  (46)
(46)
Which arrow in the following diagrams represents an endothermic phase transition? 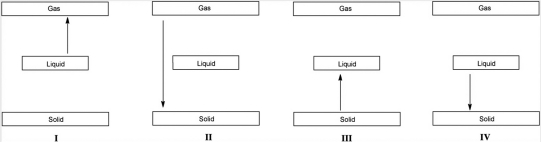
(Multiple Choice)
4.9/5  (32)
(32)
Use the following information to determine the enthalpy for the reaction shown below. CH4(g) H2O(g) CO(g) 3H2(g) H ?
2C(s) 2H2O(g) CH4(g) CO2(g) H 15.3 kJ
C(s) H2O(g) CO(g) H2(g) H 131.3 kJ
CO(g) H2O(g) CO2(g) H2(g) H 41.2 kJ
(Multiple Choice)
4.8/5  (44)
(44)
Predict the temperature change produced by burning 3.55 g benzoic acid in a bomb calorimeter that has a heat capacity of 20.12 kJ/C. The enthalpy of combustion of benzoic acid is 26.43 kJ/g.
(Multiple Choice)
4.8/5  (37)
(37)
You hold a 50 g sphere of copper in one hand and a 25 g sphere of aluminum in the other hand. If both absorb energy at the same rate, which will come to your body temperature first and why? The specific heat capacities are 0.4 J/(g 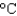 ) for copper and 0.9 J/(g
) for copper and 0.9 J/(g 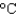 ) for aluminum.
) for aluminum.
(Multiple Choice)
4.8/5  (41)
(41)
Which arrow in the following diagrams represents an exothermic phase transition? 
(Multiple Choice)
4.9/5  (34)
(34)
Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the smallest electrostatic potential energy (i.e., smallest in magnitude)?
(Multiple Choice)
4.9/5  (35)
(35)
In terms of the enthalpy of formation, which of the following compounds is most stable relative to its elements under standard conditions?
(Multiple Choice)
4.9/5  (35)
(35)
What is the heat capacity (Cp) of a 7.5 g piece of tin if its temperature changes by 12.3C when it is supplied with 20 J from a Bunsen burner?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following substances will release the most energy during combustion in air?
(Multiple Choice)
4.8/5  (43)
(43)
You have a summer job in a lead foundry. Your task is to identify how energy efficiency can be improved. You therefore need to know the minimum amount of energy it takes to raise 1 pound of lead (454 g) from room temperature (25 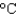 ) to its melting point (327
) to its melting point (327 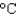 ) and then melt it. The specific heat capacity of lead is 0.159 J/(g
) and then melt it. The specific heat capacity of lead is 0.159 J/(g 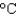 ), the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
), the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following bar charts shows the correct internal energy changes that occur in a pitcher of iced tea (system), the refrigerator (surroundings), and the universe as the iced tea in the refrigerator cools?
(Multiple Choice)
4.9/5  (35)
(35)
In an experiment, 10.0 g of ice at 20C is converted into steam with a temperature of 110 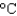 . How much energy is required for this process?
. How much energy is required for this process? 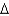 vap 2,260 J/g;
vap 2,260 J/g; 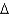 Hfus 334 J/g; cs(ice) 2.06 J/(g
Hfus 334 J/g; cs(ice) 2.06 J/(g 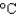 ); cs(water) 4.18 J/(g
); cs(water) 4.18 J/(g 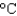 ); cs(steam) 1.99 J/(g
); cs(steam) 1.99 J/(g 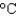 ))
))
(Multiple Choice)
4.8/5  (35)
(35)
When a 13.0 g sample of NaOH(s) dissolves in 400.0 mL of water to produce 413.0 g of solution in a constant pressure calorimeter, the temperature of the water and reaction vessel changes from 22.6C to 30.6C. Assume that the specific heat capacity of the solution is 4.20 J/(g C), and that the heat capacity of the reaction vessel is 1.00 J/C. What is the molar enthalpy of solution of sodium hydroxide (40.00 g/mol)?
(Multiple Choice)
4.8/5  (38)
(38)
The cooling system in an automobile holds 10.0 L of ethylene glycol antifreeze. How much energy is absorbed when the temperature of the ethylene glycol goes from 20 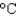 to 100
to 100  ? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g
? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g 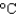 ), respectively.
), respectively.
(Multiple Choice)
4.8/5  (43)
(43)
Showing 101 - 120 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)