Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Indicate which of the following is not an element in its standard state at 25  C and 1 atm.
C and 1 atm.
(Multiple Choice)
4.8/5  (37)
(37)
Assuming that the charge of the ions remain constant in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i.e., largest in magnitude)? 
(Multiple Choice)
4.8/5  (40)
(40)
Use the following information to determine the standard enthalpy change when 1 mol of PbO(s) is formed from lead metal and oxygen gas. PbO(s)  C(graphite)
C(graphite)  Pb(s)
Pb(s)  CO(g) H
CO(g) H  107 kJ
2C(graphite)
107 kJ
2C(graphite)  O2(g)
O2(g)  2CO(g) H 222 kJ
2CO(g) H 222 kJ
(Multiple Choice)
4.9/5  (36)
(36)
In terms of the enthalpy of formation, which of the following compounds is the most unstable compared to its elements under standard conditions?
(Multiple Choice)
4.9/5  (41)
(41)
A 150 g piece of iron (CP 25.09 J/(mol  )) was heated to a temperature of 47
)) was heated to a temperature of 47  and then placed in contact with a 275 g piece of copper at 20
and then placed in contact with a 275 g piece of copper at 20  (CP 25.46 J/(mol
(CP 25.46 J/(mol  )). What was the final temperature of the two pieces of metal?
)). What was the final temperature of the two pieces of metal?
(Multiple Choice)
4.9/5  (37)
(37)
Using the following data for water, determine the final temperature when 100 g of ice at 10  is heated with 350 kJ of energy. Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2)11 J/(g K)
Specific heat capacity (liquid)
4)18 J/(g K)
Specific heat capacity (gas)
2)08 J/(g K)
is heated with 350 kJ of energy. Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2)11 J/(g K)
Specific heat capacity (liquid)
4)18 J/(g K)
Specific heat capacity (gas)
2)08 J/(g K)
(Multiple Choice)
4.9/5  (46)
(46)
At a certain elevation, the boiling point of water is 98.5  . How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol ·
. How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol ·  ), d 1.00 g/mL)
), d 1.00 g/mL)
(Multiple Choice)
4.9/5  (33)
(33)
A 15 g piece of iron (CP 25.09 J/(mol · C)) is heated to a temperature of 95C and placed into a bucket containing 4.5 gal of water (CP 75.38 J/(mol · C)) initially at 25C. Eventually, ________
(Multiple Choice)
5.0/5  (40)
(40)
Which statement A-D about a system and its surroundings is not correct?
(Multiple Choice)
4.9/5  (29)
(29)
Use the following information to determine the enthalpy for the reaction shown below. S(s) O2(g) SO2(g) H ?
S(s) 
 O2(g)
O2(g)  SO3(g)
SO3(g)  2SO2(g)
2SO2(g) 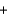 O2(g)
O2(g)  2SO3(g)
2SO3(g) 
(Multiple Choice)
4.7/5  (23)
(23)
For which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation?
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following fuels has the highest fuel value (kJ/g)?
(Multiple Choice)
4.8/5  (29)
(29)
Steam in a cylinder is compressed by a piston exerting a constant pressure of 5 atm. The volume of the cylinder decreases by 15 L and simultaneously the steam is cooled, losing 105 kJ of energy as heat. How much energy, total, was gained or lost by the steam in this process? (101.3 J 1 L atm)
(Multiple Choice)
4.9/5  (38)
(38)
Using the data shown below for water, determine in the following heating curve what region the water will be in when 50 g of ice at 25C is provided with 35 kJ of energy.  Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2.11 J/(g K)
Specific heat capacity (liquid)
4.18 J/(g K)
Specific heat capacity (gas)
2.08 J/(g K)
Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2.11 J/(g K)
Specific heat capacity (liquid)
4.18 J/(g K)
Specific heat capacity (gas)
2.08 J/(g K)
(Short Answer)
4.9/5  (37)
(37)
To cool your 250 mL of coffee at 90C, you put a 100 g metal spoon in a glass of ice water to lower its temperature to 0C, and then you put the spoon in the coffee. After thermal equilibrium has been reached, what is the final temperature of your coffee? Assume energy is exchanged only between the spoon and the coffee. The heat capacity of the metal spoon is 80 J/C. Assume the specific heat and density of coffee are the same as that of water, 4.18 J/(g C) and 1.00 g/mL.
(Short Answer)
4.7/5  (32)
(32)
Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions, and 
 (NO2)
(NO2)  33.2 kJ/mol. 2NO(g)
33.2 kJ/mol. 2NO(g)  O2(g)
O2(g)  2NO2(g) Hrxn
2NO2(g) Hrxn  114.2 kJ
114.2 kJ
(Multiple Choice)
4.8/5  (38)
(38)
Napthalene is often used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (40.18 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 7.55C when 1.250 g of napthalene was used.
(Multiple Choice)
4.9/5  (44)
(44)
State the first law of thermodynamics. Describe two situations where Esys could be negative.
(Essay)
4.8/5  (30)
(30)
During a(n) ________ process, energy is transferred from the system to the surroundings.
(Multiple Choice)
4.7/5  (45)
(45)
Showing 121 - 139 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)