Exam 10: Structure and Synthesis of Alcohols
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Reduction of a ketone with NaBH4 will result in the formation of ________.
(Multiple Choice)
4.8/5  (31)
(31)
A novice chemist wished to prepare 1-methylcyclohexane-1,4-diol from the keto alcohol shown below by treating it with the appropriate Grignard reagent. Was the chemist successful? Explain. 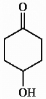
(Essay)
5.0/5  (39)
(39)
Complete the following reaction by providing a structure for the necessary starting material. 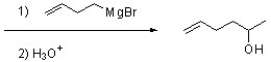
(Essay)
4.8/5  (33)
(33)
Explain how a mixture of phenol and cyclopentanol might be separated using differences in their solubility properties.
(Essay)
4.8/5  (36)
(36)
What is synthesis gas and what commercially important alcohol is prepared from it?
(Essay)
5.0/5  (32)
(32)
What sequence of reagents is needed to convert benzyl bromide into 1-phenyl-2-octanol?
(Essay)
4.8/5  (38)
(38)
Which of the following alkyl halides would be suitable to use when forming a Grignard reagent?
(Multiple Choice)
4.8/5  (43)
(43)
Phenol is more acidic than cyclohexanol because of the resonance stabilization of the phenoxide ion. Draw the proton transfer reaction that take place between sodium hydroxide and phenol. Then show the resonance structures that can be drawn for the phenoxide ion.
(Essay)
4.8/5  (33)
(33)
Provide a detailed step-by-step mechanism that would account for the formation of the product in the following reaction. 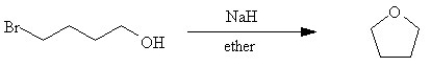
(Essay)
5.0/5  (29)
(29)
Provide a detailed, stepwise mechanism for the reaction of acetyl chloride (CH3COCl) and 2 equivalents of PhMgCl.
(Essay)
4.9/5  (35)
(35)
How would one used a Grignard-based synthesis to accomplish the following transformation?
benzyl bromide (PhCH2Br) to 3-phenylpropan-1-ol
(Essay)
4.8/5  (32)
(32)
What combination of reagents can be used in a Grignard-based synthesis of 1-propylcyclohexanol?
(Short Answer)
4.9/5  (26)
(26)
What reagent(s) are needed to complete the following reaction? 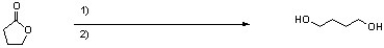
(Multiple Choice)
4.9/5  (34)
(34)
Which series of reactions would best facilitate the following conversion? 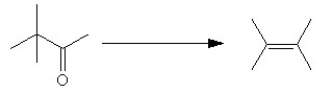
(Multiple Choice)
4.8/5  (45)
(45)
Provide the structure of the major organic product in the reaction below.
CH3CH2CH(CH3)CH2CO2CH3 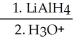 →
→
(Essay)
4.9/5  (33)
(33)
________, commonly used in automobile antifreezes, is a diol which is highly toxic if ingested.
(Short Answer)
4.7/5  (33)
(33)
Provide the structure of the major organic product in the reaction shown below.
CH3CH2CH2CHO 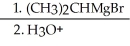 →
→
(Essay)
4.8/5  (33)
(33)
Predict the major organic product in the reaction shown below.
ethyl magnesium chloride + pent-1-yne
(Essay)
4.9/5  (35)
(35)
Showing 101 - 120 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)