Exam 19: Ionic Equilibria in Aqueous Systems
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which of the following aqueous mixtures would be a buffer system?
(Multiple Choice)
4.8/5  (31)
(31)
The solubility of aluminum hydroxide in water ______________ when dilute nitric acid is added to it.
(Multiple Choice)
4.8/5  (30)
(30)
The lab technician Anna Lytic adds 2.20 mol KOH to 1.00 L of 0.5 M Al(NO3)3. What is the concentration of aluminum ions after the aluminum nitrate has reacted with the potassium hydroxide? Kf = 3.0 × 1033 for Al(OH)4-
(Multiple Choice)
4.9/5  (37)
(37)
Write the ion product expression for calcium phosphate, Ca3(PO4)2.
(Multiple Choice)
4.9/5  (33)
(33)
What will be the effect of adding 0.5 mL of 0.1 M NaOH to 100 mL of an acetate buffer in which [CH3COOH] = [CH3COO-] = 0.5 M?
(Multiple Choice)
4.8/5  (39)
(39)
Increasing the concentrations of the components of a buffer solution will increase the buffer capacity.
(True/False)
4.8/5  (34)
(34)
Calculate the solubility of strontium fluoride, SrF2, in pure water. Ksp = 2.6 × 10-9
(Multiple Choice)
4.8/5  (41)
(41)
What is the pH of a buffer that consists of 0.45 M CH3COOH and 0.35 M CH3COONa? Ka = 1.8 × 10-5
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the solubility of silver chromate, Ag2CrO4, in 0.005 M Na2CrO4. Ksp = 2.6 × 10-12
(Multiple Choice)
4.8/5  (34)
(34)
For a diprotic acid H2A, the relationship Ka1 > Ka2 is always true.
(True/False)
4.9/5  (42)
(42)
Calculate the solubility of barium carbonate, BaCO3, in pure water. Ksp = 2.0 × 10-9
(Multiple Choice)
4.8/5  (44)
(44)
A lab technician adds 0.015 mol of KOH to 1.00 L of 0.0010 M Ca(NO3)2. Ksp = 6.5 × 10-6 for Ca(OH)2). Which of the following statements is correct?
(Multiple Choice)
4.9/5  (36)
(36)
What is the [H3O+] in a solution that consists of 1.2 M HClO and 2.3 M NaClO? Ka = 3.5 × 10-8
(Multiple Choice)
4.8/5  (40)
(40)
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak acid (0.10 mol L-1) with a strong base of the same concentration? 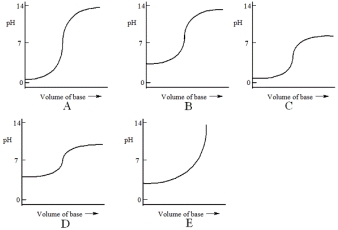
(Multiple Choice)
4.9/5  (32)
(32)
The solubility of magnesium phosphate is 2.27 × 10-3 g/1.0 L of solution. What is the Ksp for Mg3(PO4)2?
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following indicators would be the best to use when 0.050 M benzoic acid (Ka = 6.6 × 10-5) is titrated with 0.05 M NaOH?
(Multiple Choice)
4.9/5  (30)
(30)
What is the [H3O+] in a buffer that consists of 0.30 M HCOOH and 0.20 M HCOONa? For HCOOH, Ka = 1.7 × 10-4
(Multiple Choice)
4.8/5  (46)
(46)
A solution is prepared by mixing 50.0 mL of 0.50 M Cu(NO3)2 with 50.0 mL of 0.50 M Co(NO3)2. Sodium hydroxide is added to the mixture. Which hydroxide precipitates first and what concentration of hydroxide ions present in solution will accomplish the separation? Ksp = 2.2 × 10-20 for Cu(OH)2, Ksp = 1.3 × 10-15 for Co(OH)2
(Multiple Choice)
5.0/5  (40)
(40)
A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH. The following data were collected during the titration. mL NaOH added 5.00 10.00 1.00 2.00
PH 6.98 7.46 7.93 10.31
What is the Ka for HClO?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following acids should be used to prepare a buffer with a pH of 4.5?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)