Exam 19: Ionic Equilibria in Aqueous Systems
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
If the pH of a buffer solution is greater than the pKa value of the buffer acid, the buffer will have more capacity to neutralize added base than added acid.
(True/False)
4.7/5  (36)
(36)
A diprotic acid H2A has Ka1 = 1 × 10-4 and Ka2 = 1 × 10-8. The corresponding base A2- is titrated with aqueous HCl, both solutions being 0.1 mol L-1. Which one of the following diagrams best represents the titration curve which will be seen? 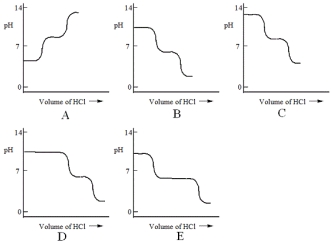
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following substances has the greatest solubility in water?
(Multiple Choice)
4.7/5  (33)
(33)
When a strong acid is titrated with a strong base, the pH at the equivalence point
(Multiple Choice)
5.0/5  (32)
(32)
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak base (0.10 mol L-1) with HCl of the same concentration? 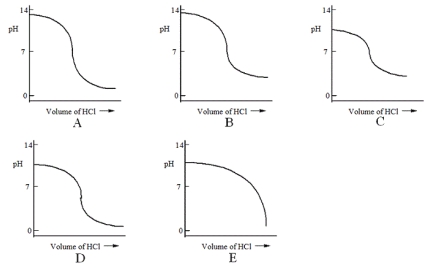
(Multiple Choice)
4.9/5  (31)
(31)
Consider the dissolution of MnS in water (Ksp = 3.0 × 10-14). MnS(s) + H2O(l)  Mn2+(aq) + HS-(aq) + OH-(aq)
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
Mn2+(aq) + HS-(aq) + OH-(aq)
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
(Multiple Choice)
4.8/5  (31)
(31)
A solution is prepared by dissolving 20.0 g of K2HPO4 and 25.0 g of KH2PO4 in enough water to produce 1.0 L of solution. What is the pH of this buffer? For phosphoric acid (H3PO4), Ka2 = 6.2 × 10-8.
(Multiple Choice)
4.9/5  (41)
(41)
Buffer solutions with the component concentrations shown below were prepared. Which of them should have the highest pH?
(Multiple Choice)
5.0/5  (40)
(40)
What is the pH of a solution that consists of 0.50 M H2C6H6O6 (ascorbic acid) and 0.75 M NaHC6H6O6 (sodium ascorbate)? For ascorbic acid, Ka = 6.8 × 10-5
(Multiple Choice)
4.8/5  (41)
(41)
The pH of blood is 7.35. It is maintained in part by the buffer system composed of carbonic acid (H2CO3) and the bicarbonate (hydrogen carbonate, HCO3-) ion. What is the ratio of [bicarbonate]/[carbonic acid] at this pH? For carbonic acid, Ka1 = 4.2 × 10-7.
(Multiple Choice)
4.8/5  (26)
(26)
The solubility of calcium chromate is 1.56 × 10-3 g/100 mL of solution. What is the Ksp for CaCrO4?
(Multiple Choice)
4.8/5  (35)
(35)
When a weak acid is titrated with a strong base, the pH at the equivalence point
(Multiple Choice)
4.8/5  (35)
(35)
What volume of 0.200 M KOH must be added to 17.5 mL of 0.135 M H3PO4 to reach the third equivalence point?
(Multiple Choice)
4.9/5  (37)
(37)
You need to prepare a buffer solution with a pH of 4.00, using NaF and HF. What ratio of the ratio of [base]/[acid] should be used in making the buffer? For HF, Ka = 7.2 × 10-4.
(Multiple Choice)
4.9/5  (37)
(37)
A 25.0-mL sample of 0.10 M C2H3NH2 (ethylamine) is titrated with 0.15 M HCl. What is the Ph of the solution after 9.00 mL of acid have been added to the amine? Kb = 6.5 × 10-4
(Multiple Choice)
5.0/5  (41)
(41)
Calculate the solubility of zinc hydroxide, Zn(OH)2, in 1.00 M NaOH. Ksp = 3.0 × 10-16 for Zn(OH)2, Kf = 3.0 × 1015 for Zn(OH)42-
(Multiple Choice)
4.9/5  (36)
(36)
A saturated solution of calcium hydroxide, Ca(OH)2, is in contact with excess solid Ca(OH)2. Which of the following statements correctly describes what will happen when aqueous HCl (a strong acid) is added to this mixture, and system returns to equilibrium? (For Ca(OH)2, Ksp = 6.5 × 10-6)
(Multiple Choice)
4.8/5  (41)
(41)
A 25.0-mL sample of 0.35 M HCOOH is titrated with 0.20 M KOH. What is the pH of the solution after 25.0 mL of KOH has been added to the acid? Ka = 1.77 × 10-4
(Multiple Choice)
4.7/5  (29)
(29)
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak diprotic acid H2A (0.10 mol L-1) with a strong base of the same concentration? 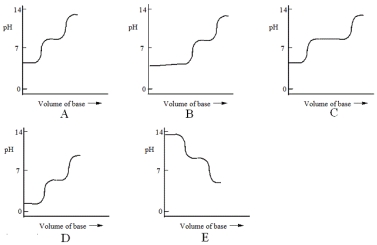
(Multiple Choice)
4.7/5  (43)
(43)
Showing 41 - 60 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)