Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
In thermodynamics,a(n)________ is defined as the object,or collection of objects,being studied.The surroundings include everything else.
(Short Answer)
4.8/5  (30)
(30)
At constant pressure and 25°C,what is ΔrH° for the following reaction 2C2H6(g)+ 7O2(g)→ 4CO2(g)+ H2O(l)
If the complete consumption of 22.7 g of C2H6 liberates -1178 kJ of heat energy?
(Multiple Choice)
4.7/5  (30)
(30)
If 50.0 g of benzene (C6H6)absorbs 2.71 kJ of energy in the form of heat at 25.0 °C,what is the final temperature of benzene? The specific heat capacity of benzene is 1.72 J/g ⋅ K.
(Multiple Choice)
4.9/5  (32)
(32)
A 18.6-g piece of gold (s = 0.129 J/(g·°C)),initially at 285.4°C,is added to 147.2 g of a liquid,initially at 27.9°C,in an insulated container.The final temperature of the metal-liquid mixture at equilibrium is 28.9°C.What is the identity of the liquid? Neglect the heat capacity of the container.
(Multiple Choice)
4.8/5  (32)
(32)
Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water. N2H4( 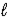 )+ O2(g)→ N2(g)+ 2 H2O(
)+ O2(g)→ N2(g)+ 2 H2O( 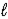 )
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 61 - 65 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)