Exam 3: Atoms, molecules, and Ions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
The mass spectrum of an element with two naturally occurring isotopes is shown below.What is the best estimate of the element's (average)atomic weight? 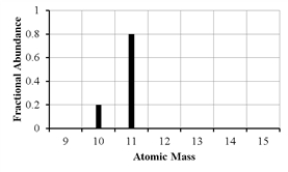
(Multiple Choice)
4.8/5  (30)
(30)
What is the symbol for an ion of an element that has 12 protons and 10 electrons?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is the correct mass percent of each element in sulfuric acid,H2SO4?
(Multiple Choice)
4.8/5  (34)
(34)
Two isotopes of a given element will have the same number of _____,but a different number of _____ in their nucleus.
(Multiple Choice)
4.7/5  (26)
(26)
The element chlorine has two stable isotopes,chlorine-35 with an atomic mass of 34.97 u and chlorine-37 with an atomic mass of 36.97 u.From the atomic weight found on the periodic table,one can conclude that _____.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the correct formula for calcium nitrate?
(Multiple Choice)
4.9/5  (41)
(41)
Elements that have the same number of protons,but differ in their number of neutrons are called _____.
(Short Answer)
4.8/5  (40)
(40)
One atomic mass unit is equal to one-twelfth of the mass of an atom of _____.
(Multiple Choice)
4.9/5  (30)
(30)
Which ionic compound has the stronger force of attraction between anions and cations: sodium bromide or potassium bromide?
(Essay)
4.8/5  (35)
(35)
What mass of aluminum contains the same number of atoms as 3.0 g of lead?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the correct molar mass of calcium chloride hexahydrate?
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the number of moles of aluminum oxide in 6.83 g of Al2O3.
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following is the correct atomic symbol for an element that has 30 neutrons and a mass number of 55?
(Multiple Choice)
4.9/5  (31)
(31)
Scandium(III)sulfite is an ionic compound formed from Sc3+ and SO32-.What is the correct way to represent the formula?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 61 - 80 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)