Exam 12: Solutions
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
What is the freezing point of a solution of 7.15 g MgCl2 in 100 g of water? Kf for water is 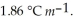
(Multiple Choice)
4.7/5  (34)
(34)
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molarity of the HCl if the solution has a density of 1.20 g mL-1.
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water. The molal freezing point depression constant ( 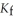 ) for water is
) for water is 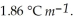
(Multiple Choice)
4.7/5  (35)
(35)
A 1.00 L sample of water contains 0.0036 g of Cl⁻ ions. Determine the concentration of chloride ions in ppm if the density of the solution is 1.00 g mL-1.
(Multiple Choice)
4.7/5  (40)
(40)
Pentane (C5H12) is mixed with 75.8 g hexane (C6H14). Calculate the vapour pressure of the resulting solution. The vapour pressures of pure pentane and hexane are 425 Torr and 151 Torr, respectively. The total solution mass is 206.6 g. Assume ideal behaviour.
(Multiple Choice)
4.8/5  (32)
(32)
A solution is 2.25% by weight NaHCO3. How many grams of NaHCO3 are in 150.0 g of solution?
(Multiple Choice)
4.9/5  (42)
(42)
Determine the solubility of CO2 in soda water at 25 °C if the pressure of CO2 is 5.2 bar. The Henry's law constant for carbon dioxide in water at this temperature is 3.4 × 10-2 mol L-1 bar-1.
(Multiple Choice)
4.9/5  (40)
(40)
A solution is prepared by dissolving 21.0 g of glycerin ( 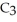
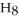
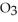 ) in 201 g of ethanol
) in 201 g of ethanol 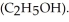 The freezing point of the solution is ________°C. The freezing point of pure ethanol is
The freezing point of the solution is ________°C. The freezing point of pure ethanol is 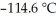 . The molal freezing point depression constant (
. The molal freezing point depression constant ( 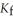 ) for ethanol is
) for ethanol is 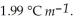 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
(Multiple Choice)
4.9/5  (40)
(40)
Calculate the mass of oxygen (in mg) dissolved in a 5.00 L bucket of water exposed to a pressure of 1.13 bar of air. Assume the mole fraction of oxygen in air to be 0.21 and the Henry's law constant for oxygen in water at this temperature to be 1.3 × 10-3 mol L-1 bar-1.
(Multiple Choice)
4.7/5  (30)
(30)
Choose the aqueous solution that has the highest boiling point. These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
(Multiple Choice)
4.8/5  (42)
(42)
What mass of CuCl2 is contained in 75.85 g of a 22.4% by mass solution of CuCl2 in water?
(Multiple Choice)
4.9/5  (40)
(40)
Calculate the vapour pressure of pure water at 65 °C if a 465 mL solution containing 123.0 g of ribose, C5H10O5, has a vapour pressure of 247.54 mbar. Assume no change in volume occurs upon dissolution.
(Multiple Choice)
4.8/5  (31)
(31)
Determine the freezing point depression of a solution that contains 30.7 g glycerin (C3H8O3, molar mass = 92.09 g mol-1) in 376 mL of water. Some possibly useful constants for water are Kf = 1.86 °C m-1 and Kb = 0.512 °C m-1.
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the freezing point of a solution of 500.0 g of ethylene glycol (C2H6O2) dissolved in 500.0 g of water. Kf = 1.86 °C m-1 and Kb = 0.512 °C m-1.
(Multiple Choice)
4.9/5  (41)
(41)
Determine the molality of a solution prepared by dissolving 0.500 moles of CaF2 in 11.5 moles H2O.
(Multiple Choice)
4.9/5  (38)
(38)
What is the expected van't Hoff factor for FeCl3? (Assume dilute conditions)
(Multiple Choice)
4.9/5  (33)
(33)
At 20 °C, a 0.389 mol L-1 aqueous solution of ammonium chloride has a density of 1.0064 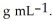 What is the mass % of ammonium chloride in the solution? The formula weight of
What is the mass % of ammonium chloride in the solution? The formula weight of 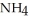 Cl is
Cl is 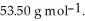
(Multiple Choice)
4.9/5  (39)
(39)
A nonelectrolyte compound with a molar mass of 648 g mol-1 was dissolved in enough water to make 250 mL of solution at 25 °C and produced an osmotic pressure of 27.4 mbar. Calculate the mass of the compound, in g, used to make this solution.
(Multiple Choice)
4.8/5  (29)
(29)
Showing 41 - 60 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)