Exam 12: Solutions
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
At 20 °C, a 2.72 mol L-1 aqueous solution of ammonium chloride has a density of 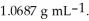 What is the molality of ammonium chloride in the solution? The formula weight of
What is the molality of ammonium chloride in the solution? The formula weight of 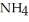 Cl is
Cl is 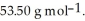
(Multiple Choice)
4.7/5  (34)
(34)
A solution of methanol and water has a vapour pressure of 248 Torr. What would you predict as the mole fractions of each component, assuming ideal behaviour? The vapour pressures of methanol and water are 256 Torr and 55.3 Torr, respectively.
(Multiple Choice)
4.9/5  (38)
(38)
Choose the aqueous solution with the lowest vapour pressure. These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
(Multiple Choice)
4.9/5  (25)
(25)
Determine the molality of an aqueous solution prepared by dissolving 0.112 moles of LiCl in 13.7 moles of H2O.
(Multiple Choice)
5.0/5  (30)
(30)
Choose the situation below that would result in an endothermic ΔsolutionH.
(Multiple Choice)
4.8/5  (47)
(47)
Calculate the mole fraction of total ions in an aqueous solution prepared by dissolving 0.400 moles of MgCl2 in 850.0 g of water.
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the volume of solution that has a vapour pressure of 25.8 mbar and contains 200.3 g of galactose, C6H12O6, at a temperature of 22.0 °C. The vapour pressure of pure water at 22 °C is 26.40 mbar, the density of water is 1.00 g mL-1, and assume no change in volume occurs upon dissolution.
(Multiple Choice)
4.9/5  (48)
(48)
Choose the aqueous solution with the highest vapour pressure. These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following compounds will be most soluble in pentane (C5H12)?
(Multiple Choice)
4.8/5  (33)
(33)
What volume of 3.00 mol L-1 CH3OH solution is needed to provide 0. 220 mol of CH3OH?
(Multiple Choice)
4.9/5  (38)
(38)
An unknown nonelectrolyte and nonvolatile compound is dissolved in enough pure water to make 229 mL of solution. The vapour pressure above the solution drops from 233.7 Torr to 229.1 Torr when 82.3 g of the compound is dissolved at constant temperature. Determine the molar mass of the unknown compound. Assume no change in volume occurs upon dissolution.
(Multiple Choice)
4.7/5  (39)
(39)
What is the molality of a glucose solution prepared by dissolving 18.0 g of glucose, C6H12O6, in 125.9 g of water?
(Multiple Choice)
4.8/5  (38)
(38)
A 125.0 mL sample of an aqueous solution at 25 °C contains 29.4 mg of an unknown nonelectrolyte compound. If the solution has an osmotic pressure of 24.4 mbar, what is the molar mass of the unknown compound?
(Multiple Choice)
4.9/5  (40)
(40)
A mixture of chloroform and acetone has a vapour pressure of 311 Torr. Calculate the mole fraction of acetone in the mixture. The vapour pressures of chloroform and acetone are 295 Torr and 332 Torr, respectively. Assume ideal behaviour.
(Multiple Choice)
4.8/5  (30)
(30)
A solution is prepared by dissolving 98.6 g of NaCl in enough water to form 875 mL of solution. Calculate the mass % of the solution if the density of the solution is 1.06 g mL-1.
(Multiple Choice)
4.7/5  (41)
(41)
At 20 °C, an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 1.0674 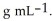 What is the molarity of ammonium chloride in the solution? The formula weight of
What is the molarity of ammonium chloride in the solution? The formula weight of 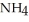 Cl is
Cl is 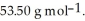
(Multiple Choice)
4.8/5  (46)
(46)
A small amount, 6.78 g, of pentane (C5H12) is mixed with 100.00 g hexane (C6H14). Calculate the vapour pressure of the resulting solution. The vapour pressures of pure pentane and hexane are 425 Torr and 151 Torr, respectively. Assume ideal behaviour.
(Multiple Choice)
4.8/5  (45)
(45)
Give the reason that antifreeze is added to a car radiator.
(Multiple Choice)
4.8/5  (32)
(32)
Showing 61 - 80 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)