Exam 12: Solutions
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
A solution of LiCl in water is 20.0 wt% LiCl. What is the mole fraction of LiCl? Assume the density of the solution is 1.00 g mL-1.
(Multiple Choice)
4.9/5  (39)
(39)
An aqueous solution has a normal boiling point of 10 3.0 °C. What is the freezing point of this solution? For water 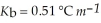 and
and 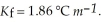
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the freezing point of a solution of 40.0 g salicylaldehyde, C7H6O2, dissolved in 800. g of benzene, C6H6. 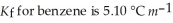 and the freezing point is 5.50 °C for benzene.
and the freezing point is 5.50 °C for benzene.
(Multiple Choice)
4.8/5  (41)
(41)
A solution is prepared by dissolving 16.4 g of benzene ( 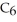
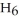 ) in 282 g of carbon tetrachloride
) in 282 g of carbon tetrachloride 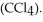 The concentration of benzene in this solution is ________ molal. The molar masses of
The concentration of benzene in this solution is ________ molal. The molar masses of 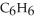 and
and 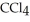 are
are 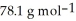 and
and 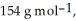 respectively.
respectively.
(Multiple Choice)
4.8/5  (28)
(28)
A solution is 0.0433 mol kg-1 LiF. What is the molarity of the solution if the density is 1.10 g mL-1?
(Multiple Choice)
4.8/5  (32)
(32)
How many moles of KF are contained in 347 g of water in a 0.175 mol kg-1 KF solution?
(Multiple Choice)
4.8/5  (32)
(32)
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molality of the HCl if the solution has a density of 1.20 g mL-1.
(Multiple Choice)
4.9/5  (42)
(42)
Determine the vapour pressure of a solution at 25 °C that contains 76.6 g of glucose (C6H12O6) in 250.0 mL of water. The vapour pressure of pure water at 25 °C is 0.03173 bar.
(Multiple Choice)
4.8/5  (40)
(40)
What is the expected van't Hoff factor for MgSO4? (Assume dilute conditions)
(Multiple Choice)
4.9/5  (37)
(37)
At a given temperature the vapour pressures of benzene and toluene are 183 mmHg and 59.2 mmHg, respectively. Calculate the total vapour pressure over a solution of benzene and toluene with Xbenzene = 0. 580.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following defines dynamic equilibrium of a solution?
(Multiple Choice)
4.7/5  (30)
(30)
A compound is found to have a molar mass of 190 g mol-1. If 0.0673 g of the compound is dissolved in enough water to make 200 mL of solution at 25 °C, what is the osmotic pressure of the resulting solution?
(Multiple Choice)
4.7/5  (35)
(35)
Calculate the volume of solution that has a vapour pressure of 31.27 mbar and contains 84.3 g of sucrose, C12H22O11, at a temperature of 25.0 °C. The vapour pressure of pure water at 25 °C is 31.7 mbar, the density of water is 1.00 g mL-1, and assume no change in volume occurs upon dissolution.
(Multiple Choice)
4.9/5  (32)
(32)
A nonelectrolyte compound with a molar mass of 862 g mol-1 was dissolved in enough water to make 100 mL of solution at 25 °C and produced an osmotic pressure of 38.4 mbar. Calculate the mass of the compound, in mg, used to make this solution.
(Multiple Choice)
4.7/5  (33)
(33)
The Henry's law constant for helium gas in water at 30 °C is 3.70 × 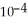 mol L-1 bar-1. When the partial pressure of helium above a sample of water is 0.450 bar, the concentration of helium in the water is ________ mol L-1.
mol L-1 bar-1. When the partial pressure of helium above a sample of water is 0.450 bar, the concentration of helium in the water is ________ mol L-1.
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the solution temperature required to produce an osmotic pressure of 1.10 mbar using 3.85 mg of a protein with a molar mass of 4183 g mol-1 that is dissolved in enough water to create 20.0 mL of solution.
(Multiple Choice)
4.9/5  (37)
(37)
A solution is prepared by dissolving 38.6 g sucrose (C12H22O11) in 495 g of water. Determine the mole fraction of sucrose if the final volume of the solution is 508 mL.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 101 - 120 of 167
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)