Exam 34: Geometric Optics
Exam 2: Motion Along a Straight Line55 Questions
Exam 3: Motion in Two or Three Dimensions59 Questions
Exam 4: Newtons Laws of Motion50 Questions
Exam 5: Applying Newtons Laws139 Questions
Exam 6: Work and Kinetic Energy109 Questions
Exam 7: Potential Energy and Energy Conservation50 Questions
Exam 8: Momentum, Impulse, and Collisions99 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics54 Questions
Exam 13: Gravitation52 Questions
Exam 14: Periodic Motion109 Questions
Exam 15: Mechanical Waves50 Questions
Exam 16: Sound and Hearing121 Questions
Exam 17: Temperature and Heat60 Questions
Exam 18: Thermal Properties of Matter41 Questions
Exam 19: The First Law of Thermodynamics55 Questions
Exam 20: The Second Law of Thermodynamics52 Questions
Exam 21: Electric Charge and Electric Field54 Questions
Exam 22: Gausss Law54 Questions
Exam 23: Electric Potential88 Questions
Exam 24: Capacitance and Dielectrics70 Questions
Exam 25: Current, Resistance, and Electromotive Force44 Questions
Exam 26: Direct-Current Circuits51 Questions
Exam 27: Magnetic Field and Magnetic Forces105 Questions
Exam 28: Sources of Magnetic Field82 Questions
Exam 29: Electromagnetic Induction51 Questions
Exam 30: Inductance88 Questions
Exam 31: Alternating Current51 Questions
Exam 32: Electromagnetic Waves Optics53 Questions
Exam 33: The Nature and Propagation of Light31 Questions
Exam 34: Geometric Optics89 Questions
Exam 35: Interference59 Questions
Select questions type
Nuclear fusion: How does the mass of the products of a nuclear fusion reaction compare to the mass of the original elements?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Nuclear reactions: For the missing product X in the reaction
neutron + 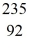 U →
U → 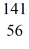 Ba + X + 3 neutrons
determine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.
Ba + X + 3 neutrons
determine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
A = 92, Z = 36, 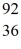 X
X
Nuclear binding energy: The iron nucleus has the greatest binding energy of any nucleus.
Free
(True/False)
4.7/5  (34)
(34)
Correct Answer:
False
Properties of the nucleus: What would be the expected radius of the nucleus of 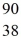 Sr?
Sr?
(Multiple Choice)
4.8/5  (39)
(39)
Radioactive decay: The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is 28 years. Suppose that samples of cobalt-60 and strontium-90 are such that they initially have the same activity (number of decays per second). What is true about the initial numbers of cobalt-60 and strontium-90 nuclei in these samples?
(Multiple Choice)
4.7/5  (33)
(33)
Biomedicine: The radioactive nuclei 60Co are widely used in medical applications. This nucleus undergoes beta decay, and the total energy of the decay process is 2.82 MeV per decay event. The half-life of this nucleus is 272 days. Suppose that a patient is given a dose of 6.9 µCi of 60Co. If all of this material decayed while in the patient's body, what would be the total energy deposited there? (1 Ci = 3.70 × 1010 decays/s)
(Multiple Choice)
4.7/5  (28)
(28)
Nuclear fission: If a 2.0-MeV neutron released in a fission reaction loses half of its energy in each moderator collision, how many collisions are needed to reduce its energy to (1/25) eV?
(Multiple Choice)
4.7/5  (33)
(33)
Nuclear binding energy: Going from medium mass nuclei to heavy nuclei, the average binding energy per nucleon
(Multiple Choice)
4.7/5  (32)
(32)
Radioactive decay: An air sample is contaminated with 15O, which has a half-life of 2.03 min. One possible way to minimize its hazard is to pass it through a long pipe to allow it to decay inside the pipe until it can be safely released into the atmosphere. If the oxygen moves at a speed of 1.1 m/s in the pipe, how long must the pipe be for the sample to have decayed to 3.0% of its original activity just as it leaves the pipe?
(Multiple Choice)
4.8/5  (32)
(32)
Nuclear reactions: In the nuclear reaction
n + 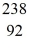 U → X + 2e-
n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.
U → X + 2e-
n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.
(Essay)
4.8/5  (30)
(30)
Radioactive dating: An archaeologist finds the 14C in a sample of 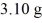 of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?
of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?
(Multiple Choice)
4.9/5  (31)
(31)
Reaction energy: Calculate the reaction energy (Q value) for the reaction 7Li + 1H → 4He + 4He, given the following masses: 7Li: 7.016005 u
1H: 1.007825 u
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
(Multiple Choice)
4.7/5  (38)
(38)
Nuclear fusion: Two deuterium nuclei,  H, fuse to produce a helium nucleus,
H, fuse to produce a helium nucleus,  H, and a neutron. A neutral deuterium atom has a mass of 2.014102 u; a neutral helium atom has a mass of 3.016030 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
H, and a neutron. A neutral deuterium atom has a mass of 2.014102 u; a neutral helium atom has a mass of 3.016030 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
(Multiple Choice)
4.9/5  (36)
(36)
Nuclear fusion: Two deuterium nuclei, 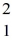 H, fuse to produce a tritium nucleus,
H, fuse to produce a tritium nucleus, 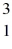 H, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016049 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
H, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016049 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
(Multiple Choice)
4.8/5  (35)
(35)
Nuclear binding energy: Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values 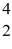 He: 4.002603 u
He: 4.002603 u 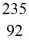 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of 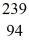 Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
(Multiple Choice)
4.8/5  (37)
(37)
Nuclear fission: An excited 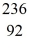 U* nucleus undergoes fission into two fragments, as shown:
U* nucleus undergoes fission into two fragments, as shown: 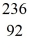 U* →
U* → 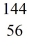 Ba +
Ba + 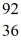 Kr The following atomic masses are known:
Kr The following atomic masses are known: 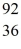 Kr: 91.926270 u
Kr: 91.926270 u 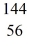 Ba: 143.922845 u
Ba: 143.922845 u 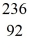 U*: 236.045563 u
What is the reaction energy, in MeV, for this process? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
U*: 236.045563 u
What is the reaction energy, in MeV, for this process? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
(Multiple Choice)
4.9/5  (40)
(40)
Biomedicine: A 70-kg laboratory technician absorbs 2.9 mJ of 0.50-MeV gamma rays in a workday. How many gamma-ray photons does the technician absorb in a workday?
(Multiple Choice)
4.8/5  (35)
(35)
Radioactive dating: An ancient rock is found to contain 40Ar gas, indicating that 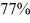 of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?
(Multiple Choice)
4.9/5  (36)
(36)
Properties of the nucleus: A certain nucleus containing 8 protons and 7 neutrons has a density ρ. Which of the following values would be closest to the expected value of the density of a nucleus having 51 protons and 69 neutrons?
(Multiple Choice)
4.8/5  (36)
(36)
Radioactive dating: The radioactivity due to carbon-14 measured in a piece of a wood from an ancient site was found to produce 20 counts per minute from a given sample, whereas the same amount of carbon from a piece of living wood produced 160 counts per minute. The half-life of carbon-14, a beta emitter, is 5730 y. The age of the artifact is closest to
(Multiple Choice)
4.7/5  (30)
(30)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)