Exam 32: Electromagnetic Waves Optics
Exam 2: Motion Along a Straight Line55 Questions
Exam 3: Motion in Two or Three Dimensions59 Questions
Exam 4: Newtons Laws of Motion50 Questions
Exam 5: Applying Newtons Laws139 Questions
Exam 6: Work and Kinetic Energy109 Questions
Exam 7: Potential Energy and Energy Conservation50 Questions
Exam 8: Momentum, Impulse, and Collisions99 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics54 Questions
Exam 13: Gravitation52 Questions
Exam 14: Periodic Motion109 Questions
Exam 15: Mechanical Waves50 Questions
Exam 16: Sound and Hearing121 Questions
Exam 17: Temperature and Heat60 Questions
Exam 18: Thermal Properties of Matter41 Questions
Exam 19: The First Law of Thermodynamics55 Questions
Exam 20: The Second Law of Thermodynamics52 Questions
Exam 21: Electric Charge and Electric Field54 Questions
Exam 22: Gausss Law54 Questions
Exam 23: Electric Potential88 Questions
Exam 24: Capacitance and Dielectrics70 Questions
Exam 25: Current, Resistance, and Electromotive Force44 Questions
Exam 26: Direct-Current Circuits51 Questions
Exam 27: Magnetic Field and Magnetic Forces105 Questions
Exam 28: Sources of Magnetic Field82 Questions
Exam 29: Electromagnetic Induction51 Questions
Exam 30: Inductance88 Questions
Exam 31: Alternating Current51 Questions
Exam 32: Electromagnetic Waves Optics53 Questions
Exam 33: The Nature and Propagation of Light31 Questions
Exam 34: Geometric Optics89 Questions
Exam 35: Interference59 Questions
Select questions type
Hydrogen atom: The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 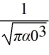 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
Angular momentum: What is the greatest magnitude of the orbital angular momentum L for an electron in a state with principal quantum number 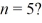
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
A
Zeeman effect: Electrons in the presence of a magnetic field transition from 4p energy states to 3d states. How many different spectral lines could be observed from these transitions?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
C
Multielectron atoms: A neutral atom has an electron configuration of 1s22s22p63s23p2. How many protons does it have in its nucleus?
(Multiple Choice)
4.8/5  (29)
(29)
Angular momentum: The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ. What is the orbital quantum number l of the electron?
(Multiple Choice)
4.8/5  (35)
(35)
Multielectron atoms: Consider the n = 9 shell.
(a) What is the largest value of the orbital quantum number, l, in this shell?
(b) How many electrons can be placed in this shell?
(Essay)
4.9/5  (41)
(41)
Lasers: In a ruby laser, an electron jumps from a higher energy level to a lower one. If the energy difference between the two levels is 1.8 eV, what is the wavelength of the emitted photon? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
5.0/5  (40)
(40)
Zeeman effect: An alkali metal atom is in the ground state. The orbital angular momentum equals zero and the spin angular momentum is entirely due to the single valence electron. A magnetic field is applied that splits the ground state energy level into two levels, 65 μeV apart.A photon, absorbed by the atom, induces a transition between the two levels. What is the wavelength of the photon? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, Bohr magneton = μB = 9.27 × 10-24 J/T, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (27)
(27)
Zeeman effect: An atom in a state with its orbital quantum number l = 1 decays to its ground state (with l = 0). A photon of wavelength 630.000 nm is emitted in the process. When the same process takes place in the presence of an intense magnetic field, the following change in the spectrum is observed. With the magnetic field present, one of the emitted lines observed now has a wavelength of 630.030 nm. Which of the following wavelengths would you expect to be also present?
(Multiple Choice)
4.8/5  (30)
(30)
Multielectron atoms: An atom has completely filled inner shells and a single valence electron in an excited p state. The filled inner shells have an orbital momentum equal to zero. A magnetic field is applied, defining the z-axis along the field. Which of the following sets of angles are possible angles between the magnetic field and the orbital angular momentum?
(Multiple Choice)
4.9/5  (31)
(31)
Lasers: How many photons per second emerge from a laser of power 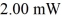 with wavelength
with wavelength 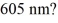 (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s)
(c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.8/5  (37)
(37)
Multielectron atoms: How many electrons can be found with principal quantum number 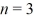 in a suitably heavy atom?
in a suitably heavy atom?
(Multiple Choice)
4.9/5  (34)
(34)
Multielectron atoms: What is the electron configuration for ground state Li, which has 3 electrons?
(Multiple Choice)
4.8/5  (25)
(25)
Quantum numbers: If the orbital quantum number is l = 4, which one of the following is a possible value for the principal quantum number n?
(Multiple Choice)
4.9/5  (43)
(43)
Multielectron atoms: An atom with 5 electrons is in its ground state. How many electrons are in its outermost shell?
(Short Answer)
4.9/5  (32)
(32)
Quantum numbers: Consider the four quantum numbers of an electron in an atom, n, l, ml, and ms. The energy of an electron in an isolated atom depends on
(Multiple Choice)
4.7/5  (39)
(39)
Multielectron atoms: What is the correct electronic configuration for ground state carbon, which has 6 electrons?
(Multiple Choice)
4.8/5  (39)
(39)
Lasers: You need 14 W of infrared laser light power with wavelength 1270 nm to bore a hole in a diamond. How many downward atomic transitions per second must occur in the laser if all of them result in light directed onto the diamond? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (35)
(35)
Lasers: The wavelength of a ruby laser is 694.3 nm. What is the energy difference between the two energy states involved in laser action? (c = 2.9979 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.6022 × 10-19 J)
(Multiple Choice)
4.9/5  (34)
(34)
Multielectron atoms: How many possible sets of quantum numbers (electron states) are there in the 5f subshell?
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)