Exam 13: Gravitation
Exam 2: Motion Along a Straight Line55 Questions
Exam 3: Motion in Two or Three Dimensions59 Questions
Exam 4: Newtons Laws of Motion50 Questions
Exam 5: Applying Newtons Laws139 Questions
Exam 6: Work and Kinetic Energy109 Questions
Exam 7: Potential Energy and Energy Conservation50 Questions
Exam 8: Momentum, Impulse, and Collisions99 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics54 Questions
Exam 13: Gravitation52 Questions
Exam 14: Periodic Motion109 Questions
Exam 15: Mechanical Waves50 Questions
Exam 16: Sound and Hearing121 Questions
Exam 17: Temperature and Heat60 Questions
Exam 18: Thermal Properties of Matter41 Questions
Exam 19: The First Law of Thermodynamics55 Questions
Exam 20: The Second Law of Thermodynamics52 Questions
Exam 21: Electric Charge and Electric Field54 Questions
Exam 22: Gausss Law54 Questions
Exam 23: Electric Potential88 Questions
Exam 24: Capacitance and Dielectrics70 Questions
Exam 25: Current, Resistance, and Electromotive Force44 Questions
Exam 26: Direct-Current Circuits51 Questions
Exam 27: Magnetic Field and Magnetic Forces105 Questions
Exam 28: Sources of Magnetic Field82 Questions
Exam 29: Electromagnetic Induction51 Questions
Exam 30: Inductance88 Questions
Exam 31: Alternating Current51 Questions
Exam 32: Electromagnetic Waves Optics53 Questions
Exam 33: The Nature and Propagation of Light31 Questions
Exam 34: Geometric Optics89 Questions
Exam 35: Interference59 Questions
Select questions type
Molar heat capacities: A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 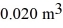 to
to 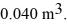 The final pressure is
The final pressure is 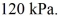 The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
First law of thermodynamics: An ideal gas with γ = 1.30 occupies 7.0 L at 300 K and 200 kPa pressure. It is compressed adiabatically to 1/7 of its original volume, then cooled at constant volume to 300 K, and finally allowed to expand isothermally to 7.0 L. How much work does the gas do during this process? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
A
First law of thermodynamics: During an adiabatic process, 20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa. The ideal gas constant is R = 8.314 J/mol ∙ K.
(a) What is the final volume of the gas?
(b) How much heat does the gas exchange during this process?
(c) What is the change in the internal (thermal) energy of the gas during this process?
Free
(Essay)
4.8/5  (29)
(29)
Correct Answer:
(a) 0.31 m3
(b) 0.00 J
(c) -32 kJ
First law of thermodynamics: In a thermodynamic process involving 7.8 moles of an ideal gas, the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3. The gas expands adiabatically to a volume of 0.080 m3. For this gas, CV = 12.27 J/mol · K, and the ideal gas constant is R = 8.314 J/mol ∙ K. Calculate the work done by the gas during this expansion.
(Short Answer)
4.9/5  (31)
(31)
First law of thermodynamics: In an isochoric process, the internal (thermal) energy of an ideal gas decreases by 50 J. How much work does the gas do during this process?
(Multiple Choice)
4.9/5  (24)
(24)
First law of thermodynamics: The pV diagram shown is for 7.50 moles of an ideal diatomic gas taken through a cycle from a to b to c. The ideal gas constant is R = 8.314 J/mol ∙ K. 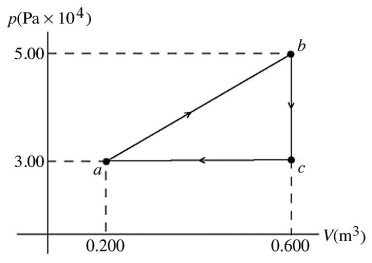 (a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?
(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?
(Essay)
4.8/5  (41)
(41)
First law of thermodynamics: An ideal gas initially at 300 K and occupying a volume of 20 L is adiabatically compressed. If its final temperature is 400 K and γ = 1.30, what is its final volume?
(Multiple Choice)
4.8/5  (27)
(27)
Types of thermodynamic processes: The process shown in the T-V diagram in the figure is an 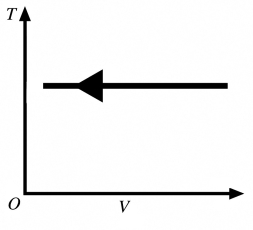
(Multiple Choice)
4.8/5  (36)
(36)
First law of thermodynamics: A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W. At what rate is the internal (thermal) energy of the gas changing?
(Multiple Choice)
4.9/5  (32)
(32)
First law of thermodynamics: When a fixed amount of ideal gas goes through an adiabatic expansion,
(Multiple Choice)
4.8/5  (32)
(32)
First law of thermodynamics: When a fixed amount of ideal gas goes through an isothermal expansion,
(Multiple Choice)
4.9/5  (25)
(25)
Molar heat capacities: The temperature of an ideal gas in a sealed 0.40- 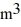 rigid container is reduced from 350 K to
rigid container is reduced from 350 K to 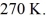 The final pressure of the gas is
The final pressure of the gas is 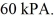 The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
(Multiple Choice)
4.7/5  (38)
(38)
First law of thermodynamics: An ideal gas increases in temperature from 22°C to 42°C by two different processes. In one process, the temperature increases at constant volume, and in the other process the temperature increases at constant pressure. Which of the following statements about this gas are correct? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (28)
(28)
Types of thermodynamic processes: When a gas undergoes an isothermal process, there is
(Multiple Choice)
4.7/5  (27)
(27)
First law of thermodynamics: During an isothermal process, 5.0 J of heat is removed from an ideal gas. How much work does the gas do during this process?
(Multiple Choice)
4.8/5  (30)
(30)
Molar heat capacities: The temperature of an ideal gas in a sealed 0.40 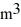 container is reduced from 400 K to
container is reduced from 400 K to 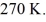 The final pressure of the gas is
The final pressure of the gas is 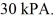 The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
(Multiple Choice)
4.9/5  (43)
(43)
Work: How much work is done by 3.00 mol of ideal gas when it triples its volume at a constant temperature of 127°C? The ideal gas constant is R = 8.314 J/mol ∙ K.
(Multiple Choice)
4.9/5  (33)
(33)
Type of thermodynamic processes: The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 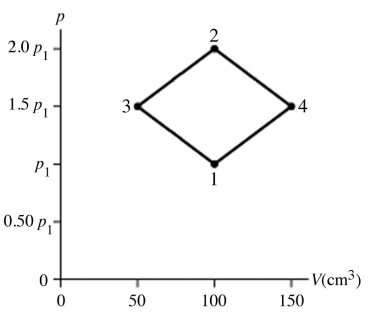
(Multiple Choice)
4.8/5  (32)
(32)
Molar heat capacities: An ideal monatomic gas cools from 455.0 K to 405.0 K at constant volume as 831 J of energy is removed from it. How many moles of gas are in the sample? The ideal gas constant is R = 8.314 J/mol ∙ K.
(Multiple Choice)
4.9/5  (22)
(22)
Molar heat capacities: An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 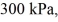 the initial volume is
the initial volume is 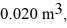 and the initial temperature is
and the initial temperature is 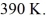 The final pressure is
The final pressure is 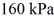 and the final temperature is
and the final temperature is 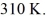 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)