Exam 30: Inductance
Exam 2: Motion Along a Straight Line55 Questions
Exam 3: Motion in Two or Three Dimensions59 Questions
Exam 4: Newtons Laws of Motion50 Questions
Exam 5: Applying Newtons Laws139 Questions
Exam 6: Work and Kinetic Energy109 Questions
Exam 7: Potential Energy and Energy Conservation50 Questions
Exam 8: Momentum, Impulse, and Collisions99 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics54 Questions
Exam 13: Gravitation52 Questions
Exam 14: Periodic Motion109 Questions
Exam 15: Mechanical Waves50 Questions
Exam 16: Sound and Hearing121 Questions
Exam 17: Temperature and Heat60 Questions
Exam 18: Thermal Properties of Matter41 Questions
Exam 19: The First Law of Thermodynamics55 Questions
Exam 20: The Second Law of Thermodynamics52 Questions
Exam 21: Electric Charge and Electric Field54 Questions
Exam 22: Gausss Law54 Questions
Exam 23: Electric Potential88 Questions
Exam 24: Capacitance and Dielectrics70 Questions
Exam 25: Current, Resistance, and Electromotive Force44 Questions
Exam 26: Direct-Current Circuits51 Questions
Exam 27: Magnetic Field and Magnetic Forces105 Questions
Exam 28: Sources of Magnetic Field82 Questions
Exam 29: Electromagnetic Induction51 Questions
Exam 30: Inductance88 Questions
Exam 31: Alternating Current51 Questions
Exam 32: Electromagnetic Waves Optics53 Questions
Exam 33: The Nature and Propagation of Light31 Questions
Exam 34: Geometric Optics89 Questions
Exam 35: Interference59 Questions
Select questions type
Uncertainty principle using ΔEΔt ≥ ħ: The energy of an electron state has an uncertainty of 0.500 eV. What is the minimum uncertainty in the lifetime of the level? (ħ = 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
A
Bohr atom: The Bohr radius of the hydrogen atom is 0.529 × 10-10 m. What is the radius of the n = 2 state?
Free
(Multiple Choice)
4.9/5  (26)
(26)
Correct Answer:
B
Matter waves: A gas of helium atoms (each of mass 6.65 × 10-27 kg) are at room temperature of 20.0°C. What is the de Broglie wavelength of the helium atoms that are moving at the root-mean-square speed? (h = 6.626 × 10-34 J ∙ s, the Boltzmann constant is 1.38 × 10-23 J/K)
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
B
Bohr atom: Light excites atomic hydrogen from its lowest level to the n = 4 level. What is the energy of the light? The energy of the lowest level is -13.6 eV.
(Multiple Choice)
4.8/5  (38)
(38)
Photoelectric effect: A metal surface has a work function of 1.50 eV. Calculate the maximum kinetic energy, in eV, of electrons ejected from this surface by electromagnetic radiation of wavelength 311 nm. (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, e = -1.60 × 10-19 C, 1 eV = 1.60 × 10-19 J)
(Short Answer)
4.8/5  (33)
(33)
Bohr atom: Suppose that in a parallel universe, the proton and electron were identical to their counterparts in our own universe EXCEPT that the electron had twice as much charge as our electron. In our present universe, the radius of the first Bohr orbit for hydrogen is a0 and the speed of an electron in that orbit is v0. In the parallel universe,
(a) what would be the radius (in terms of a0) of the first Bohr orbit for hydrogen?
(b) what would be the speed (in terms of v0) of an electron in the first Bohr orbit for hydrogen?
(Essay)
4.8/5  (34)
(34)
Matter waves: Calculate the kinetic energy (in eV) of a nonrelativistic neutron that has a de Broglie wavelength of 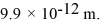 (h = 6.626 × 10-34 J ∙ s, mneutron = 1.675 × 10-27 kg, 1 eV = 1.60 × 10-19 J)
(h = 6.626 × 10-34 J ∙ s, mneutron = 1.675 × 10-27 kg, 1 eV = 1.60 × 10-19 J)
(Short Answer)
4.9/5  (36)
(36)
Compton scattering: A photon of initial wavelength 0.651 nm, after being scattered from a free electron at rest, moves off at an angle of 120° with respect to its incident direction. (mel = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s)
(a) What is the wavelength of the scattered photon?
(b) What is the energy of the scattered photon?
(Essay)
4.9/5  (37)
(37)
Uncertainty principle using ΔEΔt ≥ ħ: A certain particle's energy is measured by a detector to within 1.0 × 10-18 J. What is the minimum uncertainty we can have in its arrival time at the detector? (ħ = 1.055 × 10-34 J ∙ s)
(Multiple Choice)
4.8/5  (32)
(32)
Photoelectric effect: A stopping potential of 0.50 V is required when a phototube is illuminated with monochromatic light of wavelength 590 nm. Monochromatic light of a different wavelength is now shown on the tube, and the stopping potential is measured to be 2.30 V. What is the wavelength of this new light? (c = 3.00 × 108 m/s, e = -1.60 × 10-19 C, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (30)
(30)
Bohr atom: Light shines through atomic hydrogen gas. It is seen that the gas absorbs light readily at a wavelength of 91.63 nm. What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level. (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J; the Rydberg constant is R = 1.097 × 107 m-1.)
(Multiple Choice)
4.9/5  (31)
(31)
Uncertainty principle using ΔxΔp ≥ ħ: A nonrelativistic electron is confined to a length of 500 pm on the x-axis. What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? (ħ = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.7/5  (31)
(31)
Uncertainty principle using ΔpΔx ≥ h/2: An electron inside a hydrogen atom is confined to within a space of 0.110 nm. What is the minimum uncertainty in the electron's velocity? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
(Multiple Choice)
4.7/5  (24)
(24)
Uncertainty principle using ΔEΔt ≥ ħ: A 440-nm spectral line is produced by a transition from an excited state to the ground state. The natural line width of the spectral line is 0.020 pm. The average time the atom spends in the excited state is closest to which of the following? (ħ = 1.055 × 10-34 J ∙ s = 6.59 × 10-16 eV ∙ s)
(Multiple Choice)
4.9/5  (39)
(39)
Bohr atom: A hydrogen atom initially in the n = 6 state decays to the n = 2 state. The emitted photon is detected in a photographic plate. What is the wavelength of the detected photon? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
(Multiple Choice)
4.8/5  (34)
(34)
Uncertainty principle using ΔEΔt ≥ ħ/2: A 440-nm spectral line is produced by a transition from an excited state to the ground state. The natural line width of the spectral line is 0.020 pm. The average time the atom spends in the excited state is closest to which of the following? (ħ = 1.055 × 10-34 J ∙ s = 6.59 × 10-16 eV ∙ s)
(Multiple Choice)
4.9/5  (35)
(35)
Matter waves: A nonrelativistic electron and a nonrelativistic proton have the same de Broglie wavelength. Which of the following statements about these particles are accurate? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (37)
(37)
Uncertainty principle using ΔEΔt ≥ ħ/2: The lifetime of an excited nuclear state is 1.0 ns. What is the minimum uncertainty in the energy of this state? (ħ = 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.7/5  (27)
(27)
Photon energy: A light beam from a 2.1-mW He-Ne laser has a wavelength of 633 nm. How many photons does the laser emit in one second? (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (34)
(34)
Matter waves: Electrons emerge from an electron gun with a speed of 2.0 × 106 m/s and then pass through a pair of thin parallel slits. Interference fringes with a spacing of 2.7 mm are detected on a screen far from the double slit and fairly close to the center of the pattern. What would the fringe spacing be if the electrons were replaced by neutrons with the same speed? (mel = 9.11 × 10-31 kg, mneutron = 1.67 × 10-27 kg)
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)