Exam 17: Carboxylic Acids
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms134 Questions
Exam 3: Chemical Bonds142 Questions
Exam 4: Chemical Reactions138 Questions
Exam 5: Gases, Liquids, and Solids104 Questions
Exam 6: Solutions and Colloids157 Questions
Exam 7: Reaction Rates and Chemical Equilibrium104 Questions
Exam 8: Acids and Bases198 Questions
Exam 9: Nuclear Chemistry152 Questions
Exam 10: Organic Chemistry71 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds184 Questions
Exam 13: Alcohols, Ethers, and Thiols118 Questions
Exam 14: Chirality: the Handedness of Molecules92 Questions
Exam 15: Amines89 Questions
Exam 16: Aldehydes and Ketones102 Questions
Exam 17: Carboxylic Acids115 Questions
Exam 18: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 19: Carbohydrates103 Questions
Exam 20: Lipids132 Questions
Exam 21: Proteins128 Questions
Exam 22: Enzymes62 Questions
Exam 23: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 24: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 25: Gene Expression and Protein Synthesis129 Questions
Exam 26: Bioenergetics: How the Body Converts Food to Energy133 Questions
Exam 27: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 28: Biosynthetic Pathways67 Questions
Exam 29: Nutrition73 Questions
Exam 30: Immunochemistry132 Questions
Exam 31: Body Fluids72 Questions
Select questions type
Identify the product obtained by the decarboxylation of propanoic acid.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following compounds will have the lowest boiling point?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following notations corresponds to the fatty acid with the highest melting point?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following reagents is required in saponification?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is true of the sources of fatty acids?
(Multiple Choice)
4.9/5  (43)
(43)
In which form will carboxylic acid, RCOOH, be present in an aqueous solution with pH of the solution equal to the pKa of the acid?
(Multiple Choice)
4.9/5  (37)
(37)
Identify the group that replaces -OH in R-COOH when a carboxylic acid undergoes an esterification reaction.
(Multiple Choice)
5.0/5  (30)
(30)
Which of the following notations corresponds to the fatty acid with the lowest melting point?
(Multiple Choice)
4.8/5  (36)
(36)
Identify the correct order of pKa among the following acids: trichloroacetic acid, chloroacetic acid, and acetic acid.
(Multiple Choice)
5.0/5  (42)
(42)
Which of the following is true of the most abundant, naturally occurring fatty acids?
(Multiple Choice)
4.8/5  (37)
(37)
Identify the product formed by the decarboxylation of 3-oxobutanoic acid upon mild heating.
(Multiple Choice)
4.8/5  (37)
(37)
What product is formed when a carboxylic acid reacts with an alcohol in the presence of an acid catalyst?
(Multiple Choice)
4.7/5  (30)
(30)
In the decarboxylation of 3-oxobutanoic acid, what is the next step after a carbon dioxide molecule has been lost?
(Multiple Choice)
4.7/5  (37)
(37)
What is the product obtained when a carboxylic acid is reduced?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following is the correct structure of caproic acid?
(Multiple Choice)
4.8/5  (27)
(27)
Examine the following figure. 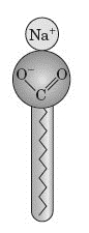 What does the image represent?
What does the image represent?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)