Exam 7: Energy and Metabolism
Exam 1: A View of Life66 Questions
Exam 2: Atoms and Molecules the Chemical Basis of Life69 Questions
Exam 3: The Chemistry of Life Organic Compounds68 Questions
Exam 4: Organization of the Cell71 Questions
Exam 5: Biological Membranes69 Questions
Exam 6: Cell Communication69 Questions
Exam 7: Energy and Metabolism73 Questions
Exam 8: How Cells Make Atp Energy-Releasing Pathways66 Questions
Exam 9: Photosynthesis Capturing Light Energy72 Questions
Exam 10: Chromosomes, Mitosis, and Meiosis66 Questions
Exam 11: The Basic Principles of Heredity78 Questions
Exam 12: Dna the Carrier of Genetic Information68 Questions
Exam 13: Gene Expression82 Questions
Exam 14: Gene Regulation77 Questions
Exam 15: Dna Technology and Genomics81 Questions
Exam 16: Human Genetics and the Human Genome75 Questions
Exam 17: Developmental Genetics83 Questions
Exam 18: Introduction to Darwinian Evolution66 Questions
Exam 19: Evolutionary Change in Populations72 Questions
Exam 20: Speciation and Macroevolution139 Questions
Exam 21: The Origin and Evolutionary History of Life67 Questions
Exam 22: The Evolution of Primates70 Questions
Exam 23: Understanding Diversity Systematics66 Questions
Exam 24: Viruses and Subviral Agents51 Questions
Exam 25: Bacteria and Archaea59 Questions
Exam 26: Protists69 Questions
Exam 27: Seedless Plants70 Questions
Exam 28: Seed Plants69 Questions
Exam 29: The Fungi69 Questions
Exam 30: An Introduction to Animal Diversity66 Questions
Exam 31: Sponges, Cnidarians, Ctenophores, and Protostomes99 Questions
Exam 32: The Deuterostomes75 Questions
Exam 33: Plant Structure Growth and Development73 Questions
Exam 34: Leaf Structure and Function76 Questions
Exam 35: Stem Structure and Transport75 Questions
Exam 36: Roots and Mineral Nutrition84 Questions
Exam 37: Reproduction in Flowering Plants81 Questions
Exam 38: Plant Developmental Responses to External and Internal Signals84 Questions
Exam 39: Animal Structure and Function an Introduction84 Questions
Exam 40: Protection Support and Movement68 Questions
Exam 41: Neural Signaling66 Questions
Exam 42: Neural Regulation67 Questions
Exam 43: Sensory Systems78 Questions
Exam 44: Internal Transport90 Questions
Exam 45: The Immune System Internal Defense79 Questions
Exam 46: Gas Exchange93 Questions
Exam 47: Processing Food and Nutrition90 Questions
Exam 48: Osmoregulation and Disposal of Metabolic Wastes111 Questions
Exam 49: Endocrine Regulation69 Questions
Exam 50: Reproduction95 Questions
Exam 51: Animal Development88 Questions
Exam 52: Animal Behavior83 Questions
Exam 53: Introduction to Ecology Population Ecology90 Questions
Exam 54: Community Ecology73 Questions
Exam 55: Ecosystems and the Biosphere91 Questions
Exam 56: Ecology and the Geography of Life81 Questions
Exam 57: Biological Diversity and Conservation Biology68 Questions
Select questions type
How is it possible for an enzyme to lower the required energy of activation for a reaction?
(Essay)
4.8/5  (35)
(35)
At a constant temperature and pH, the rate of an enzymatically catalyzed reaction is inversely proportional to the enzyme concentration.
__________________
(True/False)
4.8/5  (40)
(40)
Which refers to an organic, nonpolypeptide compound that binds to the apoenzyme and serves as a cofactor?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following two chemical equations:
A. glucose + fructose → sucrose + H2O, Δ G = +27kJ/mole (or +6.5 kcal/mole)
B. glucose + fructose + ATP → sucrose + ADP + Pi, Δ G = − 5kJ/mole (or − 1.2 kcal/mole)
What is the overall Δ G ?
(Multiple Choice)
4.7/5  (36)
(36)
According to the second law of thermodynamics, energy cannot be created nor destroyed.
__________________
(True/False)
4.7/5  (29)
(29)
Some enzymes have a receptor site that is other than the active site. This is also referred to as a(n) ____ site.
(Multiple Choice)
4.7/5  (44)
(44)
It is a fact that enzymes are highly specific and will only catalyze one or a few reactions. Can you think of a benefit that is derived from such specificity? (i.e., What would happen if most biochemical reactions were catalyzed by the same enzyme?)
(Essay)
4.9/5  (31)
(31)
In what method do competitive inhibitors inhibit biochemical reactions?
(Multiple Choice)
4.8/5  (34)
(34)
Why type of energy is represented by a positive change in G ?
(Multiple Choice)
5.0/5  (40)
(40)
Figure 7-1 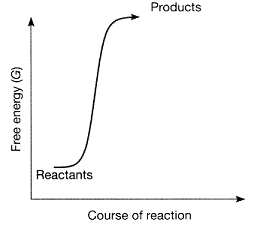 Which conclusion can be accurately derived from the accompanying figure?
Which conclusion can be accurately derived from the accompanying figure?
(Multiple Choice)
4.7/5  (43)
(43)
The cell maintains a ratio of ATP to ADP that is at the equilibrium point.
__________________
(True/False)
4.9/5  (40)
(40)
Showing 61 - 73 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)