Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
At 298 K which is larger rate of effusion or rate of diffusion?
(Short Answer)
4.8/5  (46)
(46)
The pressure in a container of gas connected to an open-end mercury manometer in which the mercury level is 22 cm lower in the side open to the atmosphere and the atmospheric pressure is
734 mm Hg is ________ mm Hg.
(Short Answer)
4.8/5  (36)
(36)
Which of the following is not equivalent to 1 atm pressure?
(Multiple Choice)
4.9/5  (43)
(43)
If the Earth's ozone (O3)layer has a total volume of 1.00 × 1020 km3,a partial pressure of 1.6 × 10-9 atm,and an average temperature of 230 K,how many ozone molecules are in the Earth's ozone layer?
(Multiple Choice)
4.8/5  (31)
(31)
A 1.75 L container filled with CO2 gas at 25°C and 225 kPa pressure springs a leak.When the container is re-sealed,the pressure is 185 kPA and the temperature is 10°C.How many moles of gas were lost?
(Multiple Choice)
4.8/5  (33)
(33)
Carbon dioxide is a gas which causes environmental concern because of the greenhouse effect.What is the approximate percentage (by volume)of CO2 in the atmosphere?
(Multiple Choice)
4.8/5  (41)
(41)
A basketball is inflated to a pressure of 1.90 atm in a 24.0°C garage.What is the pressure of the basketball outside where the temperature is - 1.00°C?
(Multiple Choice)
4.8/5  (38)
(38)
When pressure-volume measurements are made on 1.0 mol of gas at constant temperature,a plot V versus P results in a
(Multiple Choice)
4.7/5  (34)
(34)
You are given two flasks of equal volume.One contains H2 at 0°C and 1 atm while the other contains CO2 at 0°C and 2 atm.Which of the following quantities will be the same for both flasks?
(Multiple Choice)
4.9/5  (34)
(34)
A balloon filled with helium gas at 20 °C occupies 4.91 L at 1.00 atm.The balloon is immersed in liquid nitrogen at -196°C,raising the pressure to 5.20 atm.What is the volume of the balloon in the liquid nitrogen?
(Multiple Choice)
4.8/5  (38)
(38)
A 1.000 kg sample of nitroglycerine,C3H5N3O9,explodes and releases gases with a temperature of 1985°C at 1.100 atm.What is the volume of gas produced?
4 C3H5N3O9(s)→ 12 CO2(g)+ 10 H2O(g)+ 6 N2(g)+ O2(g)
(Multiple Choice)
4.7/5  (35)
(35)
Which of the noble gases should show the greatest deviation from the ideal gas law at high pressures?
(Multiple Choice)
4.9/5  (39)
(39)
A 4.00-L flask contains nitrogen gas at 25°C and 1.00 atm pressure.What is the final pressure in the flask if an additional 2.00 g of N2 gas is added to the flask and the flask cooled to -55°C?
(Multiple Choice)
4.8/5  (36)
(36)
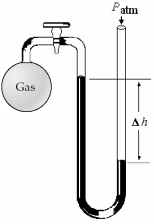 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 750 mm Hg and the difference in mercury levels,Δh,is 50 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 750 mm Hg and the difference in mercury levels,Δh,is 50 mm Hg?
(Multiple Choice)
4.9/5  (42)
(42)
What is the average speed (actually the root-mean-square speed)of a neon atom at 27°C?
(Multiple Choice)
4.8/5  (31)
(31)
An approximation of absolute zero was made from an extrapolation of
(Multiple Choice)
4.9/5  (33)
(33)
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain diffusion of a gas?
(Multiple Choice)
4.8/5  (35)
(35)
A 45.0-L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.39 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following regions of the earth's atmosphere contains the ozone layer?
(Multiple Choice)
5.0/5  (44)
(44)
Showing 21 - 40 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)