Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains CH4,flask B contains CO2,flask C contains N2.Which flask contains the largest number of molecules?
(Multiple Choice)
4.8/5  (35)
(35)
A balloon contains 0.76 mol N2,0.18 mol O2,0.031 mol He and 0.026 mol H2 at 749 mm Hg.What is the partial pressure of O2?
(Multiple Choice)
4.9/5  (30)
(30)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram. 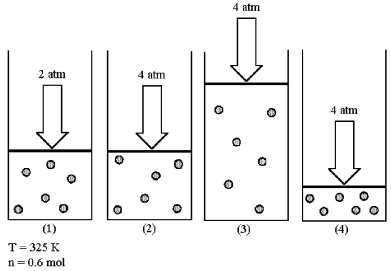 -Which diagram (2)-(4)most closely represents the result of doubling the pressure and doubling the temperature while keeping the number of moles of gas constant?
-Which diagram (2)-(4)most closely represents the result of doubling the pressure and doubling the temperature while keeping the number of moles of gas constant?
(Multiple Choice)
5.0/5  (41)
(41)
How many molecules of N2 are in a 500.0 mL container at 780 mm Hg and 135°C?
(Multiple Choice)
4.7/5  (41)
(41)
A 0.500 g sample containing Ag2O and inert material is heated,causing the silver oxide to decompose according to the following equation:
2 Ag2O(s)→ 4 Ag(s)+ O2(g)
If 13.8 mL of gas are collected over water at 27°C and 1.00 atm external pressure,what is the percentage of silver oxide in the sample? The partial pressure of water is 26.7 mm Hg at 27°C.
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following is not used to describe the condition of a gas?
(Multiple Choice)
4.8/5  (39)
(39)
A 0.286-g sample of gas occupies 125 mL at 60.cm of Hg and 25°C.What is the molar mass of the gas?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following gases has the lowest average speed at 25°C?
(Multiple Choice)
5.0/5  (33)
(33)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.  -Which diagram (2)-(4)most closely represents the result of doubling the pressure while keeping the temperature and number of moles of gas constant?
-Which diagram (2)-(4)most closely represents the result of doubling the pressure while keeping the temperature and number of moles of gas constant?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following is the principal cause of global warming?
(Multiple Choice)
4.8/5  (37)
(37)
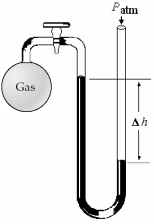 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 740 mm Hg and the difference in mercury levels,Δh,is 30 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 740 mm Hg and the difference in mercury levels,Δh,is 30 mm Hg?
(Multiple Choice)
5.0/5  (44)
(44)
At STP if 1.00 mole of gas occupies 22.4 L then 0.200 mole of gas would occupy ________ L under the same conditions.
(Short Answer)
4.8/5  (37)
(37)
A gas occupies 22.4 L at STP and 19.0 L at 100°C and 1.50 atm pressure.How many moles of gas did the system gain or lose?
(Multiple Choice)
4.8/5  (31)
(31)
One reaction that contributes to photochemical smog is shown below.Which of the species involved contains one or more unpaired electrons?
NO2(g)+ hυ →NO(g)+ O(g)
(Multiple Choice)
4.8/5  (26)
(26)
Given three cylinders containing O2 gas at the same volume and pressure.Cylinder A is at -20°C,cylinder B is at -15°F,cylinder C is at 260 K.Which cylinder contains the largest mass of oxygen?
(Multiple Choice)
4.7/5  (40)
(40)
An unusual correlation exists between the potency of an inhaled anesthetic and its ability to
(Multiple Choice)
4.8/5  (30)
(30)
Showing 81 - 100 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)