Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
When stopcock A of the open-end manometer shown below is opened,which drawing best represents the result? 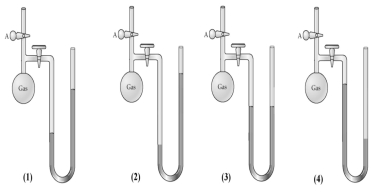
(Multiple Choice)
4.8/5  (48)
(48)
Given three cylinders containing O2 gas at the same volume and pressure.Cylinder A is at - 20°C,cylinder B is at - 15°F,cylinder C is at 260 K.Which cylinder contains the largest mass of oxygen?
(Multiple Choice)
4.9/5  (32)
(32)
At an atmospheric pressure of 745 mm Hg,what is the pressure of He gas inside a cylinder that is attached to an open-end manometer in which the level of mercury in the open side of the manometer is 25 mm Hg higher than the side that is attached to the gas cylinder?
(Short Answer)
4.8/5  (32)
(32)
One mole of gas at 25°C in a 1.0-L flask has a ________ pressure than one mole of gas at 25°C in a 5.0-L flask.
(Short Answer)
4.8/5  (31)
(31)
If mercury (density = 13.6 g/cm3)at a height of 745 mm Hg in a mercury barometer is replaced with water (density = 1.00 g/cm3),under the same conditions the height of water will be
(Multiple Choice)
4.7/5  (41)
(41)
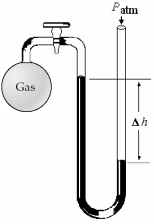 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 736 mm Hg and the difference in mercury levels,Δh,is 18 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 736 mm Hg and the difference in mercury levels,Δh,is 18 mm Hg?
(Multiple Choice)
4.9/5  (37)
(37)
At STP how many liters of NH3 can be produced from the reaction of 6.00 mol of N2 with 6.00 mol of H2?
N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.9/5  (43)
(43)
In the diagram below,nitrogen molecules are represented by unshaded spheres,oxygen molecules by gray spheres,and chlorine molecules by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of chlorine?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of chlorine?
(Multiple Choice)
4.9/5  (35)
(35)
A container filled with gas is connected to an open-end manometer that is filled with mineral oil.The pressure in the gas container is 753 mm Hg and atmospheric pressure is 724 mm.How high will the level rise in the manometer if the densities of Hg and mineral oil are 13.6 g/mL and 0.822 g/mL respectively?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following regions of the earth's atmosphere is farthest from the surface of the earth?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following gases will have the lowest rate of effusion?
(Multiple Choice)
4.8/5  (39)
(39)
A process by which gas molecules escape through a tiny hole in a membrane into a vacuum without collisions is called
(Multiple Choice)
4.9/5  (45)
(45)
The ozone molecules in the stratosphere absorb much of the ultraviolet radiation from the sun,protecting life on Earth.At a certain altitude,the temperature of the stratosphere is 240 K and the partial pressure of ozone is 1.4 × 10-7 atm.Calculate the number of ozone molecules present in 1.00 L of atmosphere at that altitude.
(Multiple Choice)
4.8/5  (32)
(32)
How many grams of XeF6 are required to react with 0.579 L of hydrogen gas at 2.46 atm and 45°C in the reaction shown below?
XeF6(s)+ 3 H2(g)→ Xe(g)+ 6 HF(g)
(Multiple Choice)
4.8/5  (38)
(38)
The action of some commercial drain cleaners is based on the following reaction:
2 NaOH(s)+ 2 Al(s)+ 6 H2O(l)→ 2 NaAl(OH)4(s)+ 3 H2(g)
What is the volume of H2 gas formed at STP when 4.32 g of Al reacts with excess NaOH?
(Multiple Choice)
4.9/5  (38)
(38)
In an open end manometer,one end of a U-tube filled with mercury is attached to a gas-filled container and the other end is open to the atmosphere.If the gas pressure in the container is less than atmospheric pressure
(Multiple Choice)
5.0/5  (29)
(29)
A gas occupies 22.4 L at STP and 14.5 L at 100°C and 2.00 atm pressure.How many moles of gas did the system gain or lose?
(Multiple Choice)
4.8/5  (28)
(28)
If NO and NH3 are allowed to effuse through a porous membrane under identical conditions,the rate of effusion for NH3 will be ________ times that of NO .
(Multiple Choice)
4.8/5  (27)
(27)
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to its ozone depletion potential.If the pressure of gaseous chloroform in a flask is 195 mm Hg at 25°C and its density is 1.25 g/L,what is the molar mass of chloroform?
(Multiple Choice)
4.9/5  (45)
(45)
Showing 41 - 60 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)