Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
At what temperature will sulfur hexafluoride molecules have the same average speed as argon atoms at 20°C?
(Multiple Choice)
4.9/5  (48)
(48)
The volume of 350.mL of gas at 25°C is decreased to 135 mL at constant pressure.What is the final temperature of the gas?
(Multiple Choice)
4.8/5  (34)
(34)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2)-(4)most closely represents the result of doubling the number of moles of gas while keeping the pressure and temperature constant? 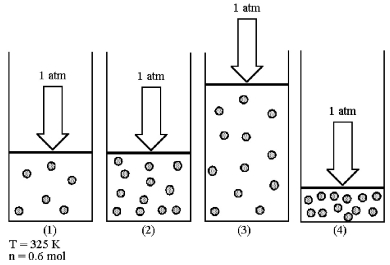
(Multiple Choice)
4.8/5  (36)
(36)
In the laboratory,hydrogen gas is usually made by the following reaction:
Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 1.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
(Multiple Choice)
4.9/5  (45)
(45)
A container filled with gas is connected to an open-end U-tube manometer that is filled with mineral oil.The pressure in the gas container is 773 mm Hg and atmospheric pressure is 754 mm Hg.What will be the difference in the levels of mineral oil in the two arms of the manometer if the densities of Hg and mineral oil are 13.6 g/mL and 0.822 g/mL respectively?
(Multiple Choice)
4.9/5  (32)
(32)
In the upper atmosphere,photochemical reactions involving organic molecules containing the elements ________ and ________ generate products that lead to ozone depletion.
(Short Answer)
4.9/5  (43)
(43)
According to the kinetic molecular theory of gases,at 298 K the average kinetic energy of O2 is ________ the average kinetic energy of CO2.
(Short Answer)
4.8/5  (36)
(36)
When 14.0 g of zinc metal reacts with excess HCl,how many liters of H2 gas are produced at STP?
(Multiple Choice)
4.9/5  (39)
(39)
Gases do not behave ideally under conditions of ________ pressure and ________ temperature.
(Short Answer)
4.7/5  (37)
(37)
According to Graham's law,the rate of effusion of a gas is inversely proportional to the ________.
(Short Answer)
4.8/5  (38)
(38)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest pressure?
(Multiple Choice)
4.8/5  (35)
(35)
Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 20 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 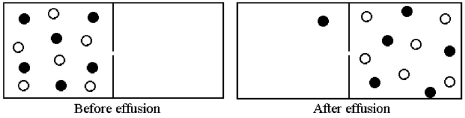
(Multiple Choice)
4.8/5  (34)
(34)
How many grams of O2 gas are there in a 5.00-L cylinder at 4.00 × 103 mm Hg and 23°C?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following gases has the highest average speed at 400K?
(Multiple Choice)
4.9/5  (38)
(38)
A lungful of air (500 mL)contains 4.1% CO2 by volume.How many grams of KO2(s)is needed to remove the CO2 from a lungful of air at STP according to the following reaction?
4 KO2(s)+ 2 CO2(g)→ 2 K2CO3(s)+ 3 O2(g)
(Multiple Choice)
4.8/5  (36)
(36)
A steel bottle contains argon gas at STP.What is the final pressure if the temperature is changed to 145°C?
(Multiple Choice)
4.8/5  (43)
(43)
An unknown gas contains 83% C and 17% H by mass.If effuses at 0.87 times the rate of CO2 gas under the same conditions.What is the molecular formula of the unknown gas?
(Multiple Choice)
4.8/5  (44)
(44)
If one mole of gas occupies 22.4 L at STP the same gas would occupy ________ than 22.4 L at 25°C and 738 mm Hg.
(Short Answer)
4.9/5  (37)
(37)
The volume of 350.mL of gas at 25°C is decreased to 125 mL at constant pressure.What is the final temperature of the gas?
(Multiple Choice)
4.9/5  (47)
(47)
What is the Celsius temperature of 100.0 g of chlorine gas in a 40.0-L container at 800 mm Hg?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 121 - 140 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)