Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest average speed?
(Multiple Choice)
4.9/5  (37)
(37)
According to the kinetic molecular theory,the pressure of a gas in a container will decrease if the
(Multiple Choice)
4.9/5  (40)
(40)
The mixing of different gases by random molecular motion with frequent collisions is called
(Multiple Choice)
4.9/5  (40)
(40)
A 0.286-g sample of gas occupies 125 mL at 60.cm of Hg and 25°C.What is the molar mass of the gas?
(Multiple Choice)
4.8/5  (36)
(36)
In the diagram below,nitrogen molecules are represented by unshaded spheres,oxygen molecules by gray spheres,and chlorine molecules by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of oxygen?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of oxygen?
(Multiple Choice)
4.8/5  (39)
(39)
What is the pressure in a gas container that is connected to an open-end U-tube manometer if the pressure of the atmosphere is 752 torr and the level of mercury in the arm connected to the container is 8.60 cm higher than the level of mercury open to the atmosphere?
(Multiple Choice)
4.9/5  (35)
(35)
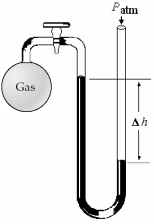 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
(Multiple Choice)
4.8/5  (31)
(31)
An "empty" aerosol can at 25°C still contains gas at 1.00 atmosphere pressure.If an "empty" can is thrown into a 475°C fire,what is the final pressure in the heated can?
(Multiple Choice)
4.8/5  (35)
(35)
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain Charles' law?
(Multiple Choice)
4.8/5  (25)
(25)
According to the kinetic molecular theory of gases,raising the temperature of a gases increases the average kinetic energy and the frequency of ________.
(Short Answer)
4.8/5  (39)
(39)
What is the volume of 10.0 g of argon gas at 157°C and 2.50 kPa pressure?
(Multiple Choice)
4.8/5  (37)
(37)
Cyanogen is a gas which contains 46.2% C and 53.8% N by mass.At a temperature of 25°C and a pressure of 750 mm Hg,1.50 g of cyanogen occupies 0.714 L.What is the molecular formula of cyanogen?
(Multiple Choice)
4.7/5  (43)
(43)
Automobile tires are typically inflated to about 30 pounds of pressure per square inch.What is the typical air pressure of a tire in kPa?
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following substances has increased markedly due to the use of fossil fuels and contributes to the greenhouse effect?
(Multiple Choice)
4.8/5  (47)
(47)
At STP the number of liters of O2 required to react with 11.2 liters of CH4 to form only CO2 and H2O is ________ liters.
(Short Answer)
4.9/5  (32)
(32)
What is the pressure in a gas container that is connected to an open-end U-tube manometer if the pressure of the atmosphere is 742 torr and the level of mercury in the arm connected to the container is 8.60 cm higher than the level of mercury open to the atmosphere?
(Multiple Choice)
4.8/5  (38)
(38)
"Equal volumes of different gases at the same temperature and pressure contain the same molar amounts" is another way of stating
(Multiple Choice)
4.9/5  (33)
(33)
Three bulbs,two of which contain different gases and one of which is empty,are connected as shown in drawing (a).Which drawing (b)- (d)best represents the system after the stopcocks are opened and the system is allowed to come to equilibrium? 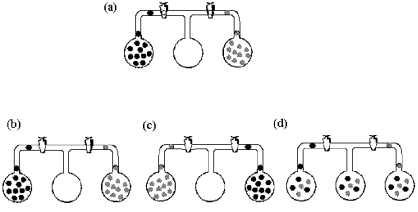
(Multiple Choice)
4.9/5  (31)
(31)
Showing 101 - 120 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)