Exam 9: Gases: Their Properties and Behavior
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
At STP how many grams of Mg are required to produce 35 mL of H2 in the reaction shown below?
Mg(s)+ 2 HCl(aq)→ H2(g)+ MgCl2(aq)
(Multiple Choice)
4.8/5  (38)
(38)
If the pressure in a gas container that is connected to an open-end U-tube manometer is 116 kPa and the pressure of the atmosphere at the open end of the tube is 752 mm Hg,the level of mercury in the tube will
(Multiple Choice)
4.9/5  (38)
(38)
What is the total pressure in a 6.00-L flask which contains 0.127 mol of H2(g)and 0.288 mol of N2(g)at 20.0°C?
(Multiple Choice)
4.8/5  (41)
(41)
According to the kinetic molecular theory of gases,the volume of the gas particles (atoms or molecules)is ________ compared to the volume of the container in which the gas particles are held.
(Short Answer)
4.8/5  (43)
(43)
How many grams of NO gas are there in a 5.00-L cylinder at 4.00 × 103 mm Hg and 23°C?
(Multiple Choice)
4.9/5  (38)
(38)
An unknown gas effuses 2.3 times faster than N2O4 at the same temperature.What is the identity of the unknown gas?
(Multiple Choice)
4.8/5  (40)
(40)
If 0.40 mol of NaN3 reacts completely in the reaction shown below,then ________ L of N2 will be produced at STP.
2 NaN3(s)→ 2 Na(s)+ 3 N2(g)
(Short Answer)
4.9/5  (34)
(34)
In the diagram below,helium atoms are represented by unshaded spheres,neon atoms by gray spheres,and argon atoms by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of helium?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of helium?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following equations represents "Boyle's law"?
(Multiple Choice)
4.8/5  (36)
(36)
An unknown gas effuses 1.73 times faster than krypton.What is the molar mass of the gas?
(Multiple Choice)
4.8/5  (42)
(42)
What is the volume of 30.0 g of argon gas at 157°C and 2.50 kPa pressure?
(Multiple Choice)
5.0/5  (33)
(33)
A gas bottle contains 0.650 mol of gas at 730 mm Hg pressure.If the final pressure is 1.15 atm,how many moles of gas were added to the bottle?
(Multiple Choice)
4.9/5  (33)
(33)
If the number of moles of gas is doubled at constant temperature and volume,the pressure of the gas
(Multiple Choice)
4.8/5  (41)
(41)
The action of some commercial drain cleaners is based on the following reaction:
2 NaOH(s)+ 2 Al(s)+ 6 H2O(l)→ 2 NaAl(OH)4(s)+ 3 H2(g)
What is the volume of H2 gas formed at STP when 6.32 g of Al reacts with excess NaOH?
(Multiple Choice)
4.9/5  (36)
(36)
A glass tube has one end in a dish of mercury and the other end closed by a stopcock.The distance from the surface of the mercury to the bottom of the stopcock is 800 mm,as indicated by the meter stick shown in the drawing below.The apparatus is at 20°C,and the mercury level in the tube is the same as that in the dish. 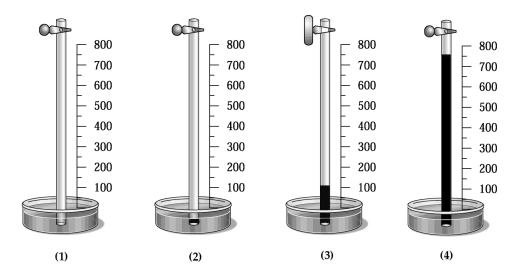 -Which drawing best shows the approximate level of the mercury in the tube when the temperature of the entire apparatus is lowered from +20°C to -20°C?
-Which drawing best shows the approximate level of the mercury in the tube when the temperature of the entire apparatus is lowered from +20°C to -20°C?
(Multiple Choice)
4.9/5  (41)
(41)
A glass tube has one end in a dish of mercury and the other end closed by a stopcock.The distance from the surface of the mercury to the bottom of the stopcock is 800 mm,as indicated by the meter stick shown in the drawing below.The apparatus is at 20°C,and the mercury level in the tube is the same as that in the dish.  -Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed,and the stopcock is reopened?
-Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed,and the stopcock is reopened?
(Multiple Choice)
4.9/5  (35)
(35)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2)-(4)most closely represents the result of doubling the temperature while keeping the pressure and number of moles of gas constant? 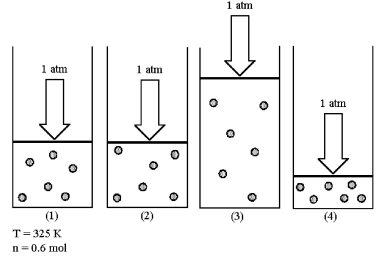
(Multiple Choice)
4.9/5  (31)
(31)
What is the average speed (actually the root-mean-square speed)of a neon atom at 27°C?
(Multiple Choice)
4.8/5  (36)
(36)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest average kinetic energy?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 141 - 160 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)