Exam 15: Principles of Chemical Equilibrium
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Write the equilibrium constant expression for the reaction:
3 Sn(s)+ 4 HNO3(aq)+ H2O(l)⇌ 3 H2SnO3(s)+ 4 NO(g)
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
Consider the following reaction:
POCl3(g)⇌ POCl(g)+ Cl2(g)with Kc = 0.450
A sample of pure POCl3(g)was placed in a reaction vessel and allowed to decompose according to the above reaction.At equilibrium,the concentrations of POCl(g)and Cl2(g)were each 0.150 M.What was the initial concentration of POCl3(g)?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
Consider the following hypothetical equilibrium reaction:
A2(g)+ B2(g)⇌ 2 AB(g) where Kc = 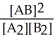 The equilibrium constant for the reaction: 2 A2(g)+ 2 B2(g)⇌ 4 AB(g)is ________.
The equilibrium constant for the reaction: 2 A2(g)+ 2 B2(g)⇌ 4 AB(g)is ________.
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
E
The equilibrium constant,Kp,equals 3.40 for the isomerization reaction:
Cis-2-butene ⇌ trans-2-butene
If a flask initially contains 0.250 bar of cis-2-butene and 0.165 bar of trans-2-butene,what is the equilibrium pressure of each gas?
(Multiple Choice)
4.8/5  (26)
(26)
For the reaction:
3 Fe(s)+ 4 H2O(g)⇌ Fe3O4(s)+ 4 H2(g)
Write the expression for Kp.
(Multiple Choice)
4.9/5  (43)
(43)
Consider the equilibrium system: N2O4(g)⇌ 2 NO2(g)for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assume that 1 mole of N2O4 and 2 moles of NO2 are introduced into a 5.0 liter container.What will be the equilibrium value of [N2O]?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following equilibrium constants-reaction types is INCORRECT?
(Multiple Choice)
4.8/5  (32)
(32)
At a certain temperature the equilibrium constant,Kc,equals 0.11 for the reaction:
2 ICl(g)⇌ I2(g)+ Cl2(g)
What is the equilibrium concentration of ICl if 0.45 mol of I2 and 0.45 mol of Cl2 are initially mixed in a 2.0 L flask?
(Multiple Choice)
4.9/5  (43)
(43)
Consider the equilibrium system: N2O4(g)⇌ 2 NO2(g)for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 10 atm at equilibrium and that initially only N2O4 was present inside the container,compute 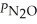 At equilibrium.
At equilibrium.
(Multiple Choice)
4.8/5  (27)
(27)
In a system in equilibrium,two opposing reactions occur at equal rates.
(True/False)
5.0/5  (33)
(33)
For the reaction CO(g)+ 3 H2(g)⇌ H2O(g)+ CH4(g),Kc = 190 at 1000 K.If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.036 M,[H2] = 0.045 M,[H2O] = 0.020,M and [CH4] = 0.031 M,in which direction will a reaction occur and why?
(Multiple Choice)
4.8/5  (32)
(32)
Write the equilibrium constant expression for the following reaction:
2 KI(aq)+ H2O2(aq)⇌ 2 KOH(aq)+ I2(aq)
(Multiple Choice)
4.9/5  (36)
(36)
For the reaction CO(g)+ 3 H2(g)⇌ H2O(g)+ CH4(g),Kc = 190 at 1000 K.If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.025 M,[H2] = 0.045 M,[H2O] = 0.025,M and [CH4] = 0.046 M,in which direction will a reaction occur and why?
(Multiple Choice)
4.8/5  (35)
(35)
Equilibrium reactions are noted by a single straight arrow for a yield sign.
(True/False)
4.8/5  (46)
(46)
For the following chemical equilibrium,Kp = 4.6 × 10-14 at 25 °C,find the value of Kc for this reaction at 25 °C.
2 Cl2(g)+ 2 H2O(g)⇌ 4 HCl(g)+ O2(g)
(Multiple Choice)
5.0/5  (40)
(40)
Kc is 1.67 × 1020 at 25 °C for the formation of iron(III)oxalate complex ion:
Fe3+(aq)+ 3C2O42-(aq)⇌ [Fe(C2O4)3]3-(aq)
If 0.0200 mol L-1 Fe3+ is initially mixed with 1.00 mol L-1 oxalate ion,what is the concentration of Fe3+ ion at equilibrium?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the reaction:
2 SO2(g)+ O2(g)⇌ 2 SO3(g),ΔrH° = -196.6 kJ/mol
The equilibrium is displaced to the left if:
(Multiple Choice)
5.0/5  (38)
(38)
Consider the following reaction occurring at 960 K:
CO2(g)+ H2(g)⇌ CO(g)+ H2O(g)
At equilibrium,the concentrations of reactants and products are: [CO2] = 0.0400 M,[H2] = 0.0220 M,[CO] = 0.0240 M,and [H2O] = 0.0190 M.This equilibrium is perturbed by adding CO2(g)to the system such that when a new equilibrium is reached,the concentration of CO2 is 0.050 M.What is the concentration of H2(g)at this equilibrium?
(Multiple Choice)
4.8/5  (28)
(28)
Consider the equilibrium system: N2O4(g)⇌ 2 NO2(g)for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 1.00 atm at equilibrium and that initially only N2O4 was present inside the container,compute 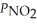 At equilibrium.
At equilibrium.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)