Exam 14: Solutions and Their Physical Properties
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
At 20 °C,a 2.32 mol L-1 aqueous solution of ammonium chloride has a density of 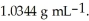 What is the molality of ammonium chloride in the solution?
The formula weight of
What is the molality of ammonium chloride in the solution?
The formula weight of 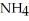 Cl is
Cl is 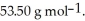
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
A
At a given temperature the vapour pressures of benzene and toluene are 183 mmHg and 59.2 mmHg,respectively.Calculate the total vapour pressure over a solution of benzene and toluene with xbenzene = 0.580.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
Nitrogen gas has a Henry's law constant k = 6.3 × 10-4 M/atm at 25 °C.The "bends" in divers results from bubbles of N2(g)being rapidly released from body fluids when a diver ascends to the surface too quickly.Which of the following would be a good substitute for N2(g)in order to make the "bends" less severe?
Free
(Multiple Choice)
4.8/5  (26)
(26)
Correct Answer:
A
A solution is prepared by dissolving 7.00 g of glycerin ( C3H8O3)in 201 g of ethanol 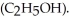 The freezing point of the solution is ________ °C.The freezing point of pure ethanol is
The freezing point of the solution is ________ °C.The freezing point of pure ethanol is 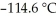 .The molal freezing point depression constant (
.The molal freezing point depression constant ( 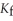 )for ethanol is
)for ethanol is 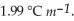 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
(Multiple Choice)
4.8/5  (31)
(31)
Moles of solute per mole of solution is a definition of ________.
(Multiple Choice)
4.9/5  (39)
(39)
Determine the osmotic pressure at 25 °C of an aqueous solution that is 0.028 M NaNO3.
(Multiple Choice)
5.0/5  (41)
(41)
What is the mole fraction of ethanol in a solution made by dissolving 29.2 g of ethanol,C2H5OH,in 53.6 g of water?
(Multiple Choice)
4.9/5  (39)
(39)
What is the osmotic pressure in mmHg of 6.00 L of a 0.108 M solution at 30 °C if three moles of ions are produced in aqueous solution for every mole of solute dissolved?
(Multiple Choice)
4.8/5  (42)
(42)
138.0 grams of ethanol (46.0 g/mol),99.0 grams of water (18.0 g/mol),and 80.0 grams of methanol (32.0 g/mol)comprise a solution.What is the mole fraction of methanol present in the solution?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following compounds has the highest aqueous solubility?
(Multiple Choice)
4.8/5  (30)
(30)
A 1.38 M solution of nitric acid (63.g/mol)in water (18.0 g/mol)has a density of 1.04 g/mL.What is the mole fraction of nitric acid in the solution?
(Multiple Choice)
4.9/5  (26)
(26)
The solubilities of ammonium bromide,NH4Br,in water at 0 °C,20 °C,and 80 °C are as follows:
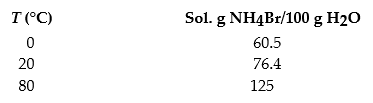 Which of the following fractional crystallization schemes would produce the highest percent yield for the recrystallization of ammonium bromide?
Which of the following fractional crystallization schemes would produce the highest percent yield for the recrystallization of ammonium bromide?
(Multiple Choice)
4.9/5  (35)
(35)
A solution composed of 5 moles of acetone (CH3COCH3,P°A = 324 mmHg)and 5 moles of chloroform (CHCl3,P° = 274 mmHg)has a vapor pressure of 236 mmHg.Which one of the following statements is completely true about this solution?
(Multiple Choice)
4.9/5  (36)
(36)
Adding a solute to a solvent lowers the freezing point of the solution compared to the pure solvent.
(True/False)
4.8/5  (33)
(33)
The vapor pressures of pure propyl alcohol and isopropyl alcohol are 21.0 mmHg and 45.2 mmHg,respectively,at 25 °C.What is the composition of the vapor in equilibrium with a propyl alcohol - isopropyl alcohol solution in which the mole fraction of propyl alcohol is 0.250?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following aqueous solutions has the highest boiling point?
(Multiple Choice)
4.8/5  (41)
(41)
The concentration unit used to calculate boiling point elevation is ________.
(Multiple Choice)
4.8/5  (23)
(23)
An unsaturated solution will have some undissolved solid in the bottom of the container.
(True/False)
4.8/5  (27)
(27)
A solution is prepared by adding 30.00 g of lactose (milk sugar)to 110.0 g of water at 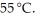 The partial pressure of water above the solution is ________ Torr.The vapour pressure of pure water at 55 °C is 0.1553 bar.The MW of lactose is
The partial pressure of water above the solution is ________ Torr.The vapour pressure of pure water at 55 °C is 0.1553 bar.The MW of lactose is 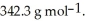
(Multiple Choice)
4.7/5  (42)
(42)
The stronger the forces between solute and solvent molecules,the more exothermic the solution process.
(True/False)
4.9/5  (37)
(37)
Showing 1 - 20 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)