Exam 28: Chemistry of the Living State
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What is the common name of the following glycose shown in Fischer notation? 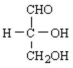
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
A
Choose the INCORRECT statement about metabolism.
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
B
What is the absolute configuration of the following amino acids? 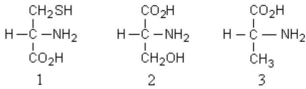
Free
(Multiple Choice)
4.8/5  (27)
(27)
Correct Answer:
A
A mixture of the three amino acids shown below was buffered to pH 7.0 and placed in the center of an electric field with the cathode to the left and the anode to the right.From left to right,what would be the positional order of the amino acids after sufficient time to allow migration? 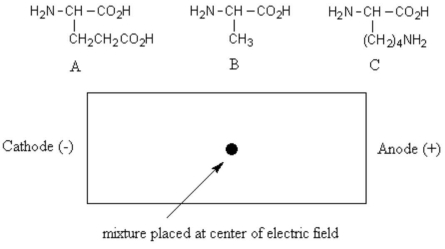
(Multiple Choice)
4.9/5  (32)
(32)
Choose the INCORRECT prediction.By careful inspection of the formula of lactic acid,CH3CH(OH)COOH,one would predict:
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following are unimportant for tertiary structure of the proteins?
(Multiple Choice)
4.7/5  (44)
(44)
What is the chemical structure of the tetrapeptide (H2N-end)-Ala-Cys-Asp-Glu?
(Short Answer)
4.9/5  (37)
(37)
What four elements make up approximately 96% of the total human body mass?
(Multiple Choice)
4.8/5  (38)
(38)
When the equation for the oxidation of glucose is balanced,the coefficients are: ________.
C6H12O6 + O2 → CO2 + H2O
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following readily dissolve in nonpolar solvents?
(Multiple Choice)
4.8/5  (31)
(31)
Apart from bringing the reactants close together,how does the binding of substrates in the active site of the enzyme serve to catalyze a reaction?
(Multiple Choice)
5.0/5  (38)
(38)
Cellulose and starch are both polymers of glucose units,but most animals can only use starch as a source of carbohydrate in their diets.Why are most animals incapable of digesting cellulose?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)