Exam 7: Thermochemistry
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Combine the reactions:
P4(s)+ 6 Cl2(g)→ 4 PCl3(g)ΔrH°298 = -1225.0 kJ/mol
PCl3(g)+ 3 H2O(l)→ H3PO3(aq)+ 3 HCl(g)= -853.5 kJ/mol
2 H2(g)+ O2(g)→ 2 H2O(l)= -571.5 kJ/mol
H2(g)+ Cl2(g)→ 2 HCl(g)= -184.9 kJ/mol
To obtain the reaction: P4(s)+ 6 H2(g)+ 6 O2(g)→ 4 H3PO3(aq).
What is the value of ΔrH°298 for this reaction?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
A 12-inch diameter ball of pure cobalt metal at 225 °C was placed in 10.0 gal.of water at 15.0 °C.What is the final temperature of the water? Assume no heat is lost to the surroundings.(specific heat for cobalt is 0.421 J/g °C,density of cobalt = 8.862 g/cm3,1 gal.= 3.785 L,1 in = 2.54 cm,V(sphere)= 4/3 πr3)
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
Calculate the enthalpy change for the following reaction at 25 °C.The value of ΔfH° in kJ/mol is given below each species:
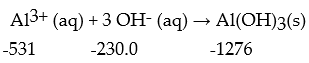
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
B
The standard heat of formation of solid ammonium bromide is -270.8 kJ/mol.Write the chemical equation for the reaction to which this value applies.
(Short Answer)
4.8/5  (37)
(37)
A 50.0 g sample of liquid water at 25.0 °C is mixed with 23.0 g of water at 89.0 °C.The final temperature of the water is ________ °C.
(Multiple Choice)
4.8/5  (29)
(29)
The complete combustion of 1 mole of nitrobenzene,C6H5NO2,in a bomb calorimeter liberates 3088 kJ of heat and increases the temperature of the calorimeter assembly by 140.0 °C.What is the heat capacity of this bomb calorimeter?
(Multiple Choice)
4.9/5  (34)
(34)
The final temperature when 150 mL of water at 90.0 °C is added to 100.0 mL of water at 30.0 °C is around ________.
(Multiple Choice)
4.7/5  (39)
(39)
Lead,water,sulfur and arsenic have specific heats of 0.128,4.18,0.706,and 0.329 J g-1 °C-1,respectively.Which of the following would need the smallest quantity of heat to change the temperature of 5 g by 10 °C?
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the standard enthalpy of formation of Cl-(aq),given the following thermochemical data at 1 atm,and knowing that the standard enthalpies of formation of H2(g)and H+(aq)are both zero.
HCl(g)→ H+(aq)+ Cl-(aq)ΔrH° = -75.15 kJ/mol
H2(g)+ Cl2(g)→ 2HCl(g)ΔrH° = -184.62 kJ/mol
(Multiple Choice)
4.9/5  (34)
(34)
When 2.50 g of Ba(s)is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00 °C to 40.32 °C.If the specific heat of the solution is 4.18 J g-1°C-1 ,calculate
 For the reaction,as written.
Ba(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ H2(g)
For the reaction,as written.
Ba(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ H2(g)
 = ?
= ?
(Multiple Choice)
4.8/5  (33)
(33)
How much heat is absorbed/released when 40.00 g of NH3(g)reacts in the presence of excess O2(g) to produce NO(g)and H2O(l)according to the following chemical equation?
4 NH3(g)+ 5 O2(g)→ 4 NO(g)+ 6 H2O(l)
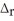 H° = 1168 kJ
H° = 1168 kJ
(Multiple Choice)
4.7/5  (38)
(38)
What is the final temperature in the bomb calorimeter if 1.785 grams of benzoic acid (C6H5COOH)is combusted in a calorimeter with a heat capacity of 5.02 kJ/ °C and initial temperature of 24.62 °C? The heat of combustion of benzoic acid is -26.42 kJ/g.
(Multiple Choice)
4.8/5  (22)
(22)
The combustion of titanium with oxygen produces titanium dioxide:
Ti(s)+ O2(g)→TiO2 (s)
When 2.060 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00 °C to 91.60 °C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJK-1 .The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ mol-1.
(Multiple Choice)
4.8/5  (27)
(27)
What is the work done in joules by the system when H2 expands against a constant pressure of 75 atm at 45.3 °C? The change in volume is 24.0 L.
(Multiple Choice)
4.9/5  (36)
(36)
What is the reaction for the standard enthalpy of formation for HCl(g)?
(Multiple Choice)
4.9/5  (31)
(31)
Calculate the amount of heat absorbed by a 40.0 g sample of water at 21 °C when the temperature is raised to 35 °C.
(Multiple Choice)
5.0/5  (33)
(33)
When power was turned off to a 30.0 gal.water heater,the temperature of the water dropped from 75.0 °C to 22.5 °C.How much heat was lost to the surroundings?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)