Exam 1: Matter: Its Properties and Measurement
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
How many litres of air are in a room that measures 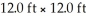 And has an 8.00 ft ceiling?
And has an 8.00 ft ceiling?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
A
What is the answer to the correct number of significant figures of the following calculation? 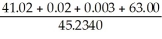
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
B
Which of the following is a chemical change?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
E
At what temperature will the numerical values of the Celsius and Fahrenheit scales be the same?
(Multiple Choice)
4.9/5  (25)
(25)
An elephant's bathtub is 8.0 feet long,2.5 yards wide,and 70 inches deep.What is the capacity of the elephant's bathtub in U.S.quarts?
(Multiple Choice)
4.8/5  (46)
(46)
The following measurements were made by a group of students using the same balance and a 25.00 gram weight.
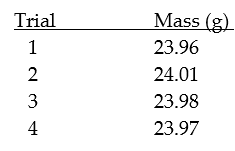 The data would be considered:
I.accurate and precise
II.accurate but not precise
III.precise but not accurate
IV.neither precise nor accurate
V.evidence of a systematic error
VI.evidence of large random errors
The data would be considered:
I.accurate and precise
II.accurate but not precise
III.precise but not accurate
IV.neither precise nor accurate
V.evidence of a systematic error
VI.evidence of large random errors
(Multiple Choice)
4.8/5  (36)
(36)
From ammonia gas,one can obtain two different gases,each of which is a pure substance.Using only this information,it can be said with certainty that:
(Multiple Choice)
4.7/5  (24)
(24)
The temperature in the core of the warp drive of the starship Enterprise is supposedly 3 million (3.00 × 106)degrees Celsius.What is this in degrees Fahrenheit?
(Multiple Choice)
4.7/5  (39)
(39)
The definition of pH is pH = -log[H3O+],where [H3O+] is the hydronium ion concentration.
If [H3O+] = 1.2 × 10-5,the pH,expressed to the correct number of significant figures,is:
(Multiple Choice)
4.9/5  (42)
(42)
It takes light one second to travel 2.998 × 108 m.How many kilometers does light travel in a day?
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following defines or expresses the concept of accuracy?
(Multiple Choice)
4.9/5  (33)
(33)
A jewelry alloy has a density of 12.412 g/mL and is 75.0% gold by weight.If 522 g of gold are available,what volume of this alloy can be produced?
(Multiple Choice)
4.9/5  (36)
(36)
A piece of metal ore weighs 8.25 g.When a student places it into a graduated cylinder containing water,the liquid level rises from 21.25 mL to 26.47 mL.What is the density of the ore?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)