Exam 5: Introduction to Reactions in Aqueous Solutions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Which of the following represents a disproportionation reaction?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
In which of the following pairs is the oxidation number for the underlined element INCORRECT?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
C
Which of the following represents a neutralization reaction?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 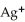 (aq)+
(aq)+ 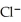 (aq)→ AgCl(s)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.366 mol L-1
(aq)→ AgCl(s)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.366 mol L-1 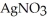 (aq)solution to completely precipitate the silver?
(aq)solution to completely precipitate the silver?
(Multiple Choice)
4.8/5  (33)
(33)
Gold does not react with either nitric acid or hydrochloric acid but with a combination of both called aqua regia.Identify the oxidizing agent in the following equation:
Au(s)+ 4 H+(aq)+ NO3-(aq)+ 4 Cl-(aq)→ [AuCl4]-(aq)+ 2 H2O(l)+ NO(g)
(Multiple Choice)
4.8/5  (43)
(43)
Which of the compounds H2C2O4,Ca(OH)2,KOH,and HI behave as bases when they are dissolved in water?
(Multiple Choice)
5.0/5  (29)
(29)
Identify the reducing agent in the following reaction:
2 NO2(g)+ 7 H2(g)→ 2 NH3(g)+ 4 H2O(g)
(Multiple Choice)
4.7/5  (33)
(33)
When 280 mL of 1.50 × 10-4 mol L-1 hydrochloric acid is added to 105 mL of 1.75 × 10-4 mol L-1 Mg(OH)2,the resulting solution will be:
(Multiple Choice)
4.8/5  (35)
(35)
The mixing of which pair of reactants will result in a precipitation reaction?
(Multiple Choice)
4.7/5  (34)
(34)
The mixing of which pair of reactants will result in a precipitation reaction?
(Multiple Choice)
4.8/5  (33)
(33)
Assuming that the proposed aqueous reactants are mixed in stoichiometric ratios,and at moderate concentrations,which of the following is least likely to represent an observable (e.g. ,gas evolution or precipitation)reaction?
(Multiple Choice)
4.8/5  (31)
(31)
A piece of iron wire weighing 1.63 g is converted to Fe2+(aq)and requires 21.9 mL of KMnO4 solution for its titration.What is the molarity of the KMnO4 solution? The reaction occurring during the titration is:
5 Fe2+ (aq)+ MnO4- (aq)+ 8 H+ (aq)→ 5 Fe3+ (aq)+ Mn2+ (aq)+ 4 H2O
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following would have the strongest tendency of producing OH- ions in an aqueous solution?
(Multiple Choice)
4.9/5  (33)
(33)
Trace amounts of oxygen gas can be "scrubbed" from gases using the following reaction:
4 Cr2+(aq)+ O2(g)+ 4 H+(aq)→ 4 Cr3+(aq)+ 2 H2O(l)
Which of the following statements is true regarding this reaction?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following solutions has the highest fluoride concentration?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 1 - 20 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)