Exam 4: Chemical Reactions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
When 11.0 g of calcium metal is reacted with water,5.00 g of calcium hydroxide is produced.Using the following balanced equation,calculate the percent yield for the reaction.
Ca(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ H2(g)
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
B
(aq)indicates that the compound is dissolved in alcohol.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
How many grams of a solid mixture containing strontium chloride would one need to make 558 mL of a 0.100 M strontium chloride solution,if the solid mixture contains 58.6% strontium chloride by weight?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
B
How many grams of H2 gas can be produced by the reaction of 54.0 grams of Al(s)with an excess of dilute hydrochloric acid in the reaction shown below?
2 Al(s)+ 6 HCl(aq)→ 2 AlCl3(aq)+ 3 H2(g)
(Multiple Choice)
4.8/5  (36)
(36)
The reactant that is in excess determines the amount of products formed.
(True/False)
4.7/5  (32)
(32)
If the percent yield for the following reaction is 75.0%,and 45.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq),are produced?
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
(Multiple Choice)
4.8/5  (34)
(34)
Lithium and nitrogen react to produce lithium nitride:
6 Li(s)+ N2(g)→ 2Li3N(s)
How many moles of N2 Are needed to react with 0.500 mol of lithium?
(Multiple Choice)
4.7/5  (40)
(40)
How many grams of NaOH (MW = 40.0 g mol-1)are there in 250.0 mL of a 0.275 mol L-1 NaOH solution?
(Short Answer)
4.9/5  (35)
(35)
What is the concentration of HCl in the final solution when 65 mL of a 12 mol L-1 HCl(aq)solution is diluted with pure water to a total volume of 0.15 L?
(Multiple Choice)
4.9/5  (36)
(36)
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:
2NaN3(s)→ 2 Na(s)+ 3N2(g)
How many grams of sodium azide are required to produce 33.0 g of nitrogen?
(Multiple Choice)
4.8/5  (38)
(38)
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
CaO(s)+ H2O(l)→ Ca(OH)2(s)
In a particular experiment,a 5.00 g sample of CaO is reacted with excess water and 6.11 g of Ca(OH)2 Is recovered.What is the percent yield in this experiment?
(Multiple Choice)
4.8/5  (32)
(32)
In the reaction: 4 Zn + K2Cr2O7 + 7 H2SO4→ 4 ZnSO4 + 2 CrSO4 +K2SO4 + 7 H2O,if the extent of reaction,x,is 0.0393,how many grams of potassium dichromate is required?
(Multiple Choice)
4.8/5  (35)
(35)
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:
2NaN3(s)→ 2 Na(s)+ 3N2(g)
How many moles of N2 Are produced by the decomposition of 2.88 mol of sodium azide?
(Multiple Choice)
4.8/5  (38)
(38)
A student dissolved 4.00 g of Co(NO3)2 in enough water to make 100 mL of stock solution.He took 4.00 mL of the stock solution and then diluted it with water to give 275 mL of a final solution.How many grams of NO3- ion are there in the final solution?
(Multiple Choice)
4.8/5  (38)
(38)
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
CaO(s)+ H2O(l)→ Ca(OH)2
A 4.50 g sample of CaO is reacted with 4.34 g of H2O
O.How many grams of water remain after the reaction is complete?
(Multiple Choice)
4.8/5  (30)
(30)
What is the molarity of a sucrose solution (C12H22O11)if 110.0 g of a 92.0% pure solid is dissolved per 250.0 mL of water?
(Multiple Choice)
4.8/5  (40)
(40)
In the reaction: 4 Zn + K2Cr2O7 + 7 H2SO4→ 4 ZnSO4 + 2 CrSO4 +K2SO4 + 7 H2O,if 25.4 g of zinc sulfate is to be made,how many grams of potassium dichromate is required?
(Multiple Choice)
4.9/5  (34)
(34)
How many grams of iron(II)sulfide is required to make 225 g of sulfuric acid,H2SO4? The reactions involved are given below.
4 FeS + 7O2 → 2 Fe2O3 + 4 SO2
2 SO2 + O2 → 2 SO3
SO3 + H2O → H2SO4
(Multiple Choice)
4.8/5  (39)
(39)
How many millilitres of 0.132 mol L-1 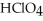 Solution are needed to neutralize 50.00 mL of 0.0789 mol L-1 NaOH?
Solution are needed to neutralize 50.00 mL of 0.0789 mol L-1 NaOH?
(Multiple Choice)
4.8/5  (33)
(33)
Sodium metal and water react to form hydrogen and sodium hydroxide.If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide,what mass of water was involved in the reaction?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)