Exam 13: Spontaneous Change: Entropy and Gibbs Energy
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Ethanol ( 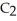
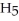 OH)melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
OH)melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
What is ΔrH° for a reaction that has Kp = 1.456 at 273 K and Kp = 14.2 at 298 K?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
For CO(g)+ H2(g)→ H2CO(g),ΔrH° = -5.36 kJ/mol,and ΔrS° = -109.8 J/mol K.What is the temperature at which Keq is 1.0 × 10-2?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
D
For the reaction: CO(g)+ 2 H2(g)→ CH3OH(g)
Kp = 91.4 at 350 K and Kp = 2.05 × 10-4 at 298 K.
What is the value of ΔrH°?
(Multiple Choice)
5.0/5  (30)
(30)
How much heat is released when 85.0 g of steam at 100.0 °C is cooled to ice at -15.0 °C? The enthalpy of vaporization of water is 40.67 kJ mol-1,the enthalpy of fusion for water is 6.01 kJ mol-1,the molar heat capacity of liquid water is 75.4 J mol-1 °C-1,and the molar heat capacity of ice is 36.4 J mol-1 °C-1.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following substances under equal conditions and in the same phase has the greatest molar entropy?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the listed reactions would couple with the following reaction to produce silver?
Ag2O(s)→ 2 Ag(s)+ 1/2 O(g)ΔrG° = 11.2 kJ/mol
(Multiple Choice)
4.8/5  (41)
(41)
The equilibrium constant for the reaction below is 7.2 × 10-4 at 298 K and 1 atm.
HNO2(aq)+ H2O(l)⇌ NO2-(aq)+ H3O+(aq)
Which of the below statements is TRUE under the following conditions:
[HNO2(aq)] = 1.0 M and [NO2-(aq)] = [H3O+(aq)] = 1.0 × 10-5 M
(Multiple Choice)
4.8/5  (34)
(34)
The normal melting and boiling points of SO2 are 198 and 263 K,respectively.For a plot of the standard molar entropy of SO2 versus temperature,which of the following is INCORRECT?
(Multiple Choice)
4.9/5  (45)
(45)
The Gibbs energy change for a reaction is -298 kJ.The reaction is therefore:
(Multiple Choice)
4.7/5  (41)
(41)
For the reaction PCl5 (g)⇌ PCl3 (g)+ Cl2 (g),Kc = 0.0454 at 261 °C.If a vessel is filled with theses gases such that the initial concentrations are [PCl5] = 0.2 M,[PCl3] = 0.2 M,and [Cl2] = 2.25 M,which of the following statements is TRUE?
(Multiple Choice)
4.7/5  (43)
(43)
Consider the following reaction:
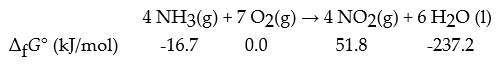 What is ΔrG° for this reaction in kJ?
What is ΔrG° for this reaction in kJ?
(Multiple Choice)
4.9/5  (31)
(31)
Standard Gibbs energy of formation requires the reactants be compounds in their standard state.
(True/False)
4.9/5  (30)
(30)
Consider the reaction: AB(g)→ A(g)+ B(g)
(Keq = 0.729 at 20 °C ΔrH° = 45.9 kJ/mol)
What is Keq for this reaction at 377 K?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following combinations of signs for △H and △S will always result in a reaction being non spontaneous?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following processes would result in a decrease in system entropy?
(Multiple Choice)
4.8/5  (30)
(30)
Consider the following reaction:
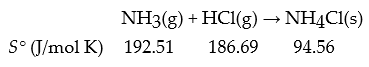 What is ΔrS° for this reaction in J/mol K?
What is ΔrS° for this reaction in J/mol K?
(Multiple Choice)
4.8/5  (34)
(34)
As the activity a of a substances increases so does its chemical potential m.
(True/False)
4.7/5  (36)
(36)
Calculate the temperature for which Keq for a reaction is 1.04 × 103 where ΔrH° = -83.2 kJ/mol and ΔrS° = -246 J/mol K.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)