Exam 20: Chemical Kinetics
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What is the order of reaction for the following reaction: Rate = k[A]-1/2 [B]1/2?
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
B
Energy of activation has no effect on reaction rate.
Free
(True/False)
4.8/5  (32)
(32)
Correct Answer:
False
The decomposition of dinitrogen pentoxide is described by the chemical equation
2 N2O5(g)→ 4 NO2(g)+ O2(g).
If the rate of disappearance of N2O5 is equal to 1.60 mol min-1 at a particular moment,what is the rate of appearance of NO2 at that moment?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
C
Data for the reaction A + B → C are given below.Find the rate constant for this system. 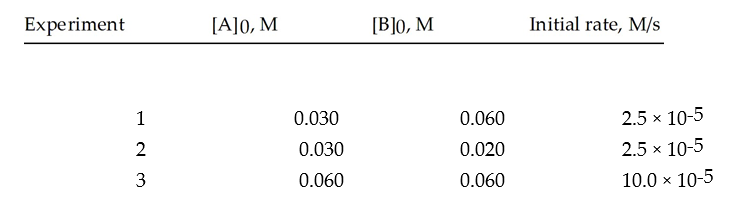
(Multiple Choice)
4.8/5  (38)
(38)
A reaction is first order.If its initial rate is 0.0200 M/s and 25.0 days later its rate is 6.25 × 10-4 M/s,then its half-life is ________.
(Multiple Choice)
4.9/5  (44)
(44)
Activation energy is:
I.the minimum kinetic energy required for each molecule in a collision to produce a reaction.
II.the minimum total kinetic energy required for the molecules in a collision to produce a reaction.
III.a factor in determining the rate of a reaction.
IV.high for fast reactions.
(Multiple Choice)
4.9/5  (24)
(24)
According to the collision theory in gaseous molecules,collision frequency is ________ and the rate of reaction is ________ because ________.
(Multiple Choice)
4.8/5  (40)
(40)
The rate of disappearance of HBr in the gas phase reaction
2 HBr(g)→ 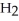 (g)+
(g)+ 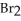 (g) Is 0.130 mol L-1
(g) Is 0.130 mol L-1 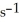 At 150 °C.The rate of reaction is ________ mol L-1
At 150 °C.The rate of reaction is ________ mol L-1 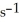 .
.
(Multiple Choice)
4.9/5  (29)
(29)
What is the overall reaction order for the reaction that has the rate law Rate = k[Cl ][N]2?
(Multiple Choice)
4.8/5  (30)
(30)
For a reaction Rate = k[A][B]2,what factor will keep k unchanged?
(Multiple Choice)
4.9/5  (25)
(25)
For the second order reaction A → products,the following data are obtained:
[A] = 3.024 M,t = 0 min
[A] = 2.935 M,t = 1.0 min
[A] = 2.852 M,t = 2.0 min
What is the rate constant,k?
(Multiple Choice)
4.7/5  (38)
(38)
In a second order reaction:
I.the sum of the exponents in the rate law is equal to two.
II.at least one of the exponents in the rate law is a two.
III.the half-life is dependent on the initial concentration of the reactant species.
IV.the half-life is independent of the initial concentration of the reactant species.
V.k can be expressed as M-2s-1 or M-2min-1.
(Multiple Choice)
4.7/5  (34)
(34)
For the second order reaction A → products,the following data are obtained:
[A] = 1.512 M,t = 0 min
[A] = 1.490 M,t = 1.0 min
[A] = 1.469 M,t = 2.0 min
What is the rate constant,k,for the reaction?
(Multiple Choice)
4.8/5  (48)
(48)
What is the rate law for the following reaction and its mechanism?
2 O3 → 3 O2 (overall reaction)
O3 → O2 + O∙ (slow)
O∙ + O3 → 2 O2 (fast)
(Multiple Choice)
4.8/5  (29)
(29)
In the Arrhenius equation,ln k = -Ea/RT + ln A,the symbol A denotes:
(Multiple Choice)
4.8/5  (36)
(36)
The isomerization of methylisonitrile to acetonitrile
![The isomerization of methylisonitrile to acetonitrile NC(g)→ CN(g) Is first order in NC.The half-life of the reaction is 5.20 × 10<sup>1</sup> s at 545 K.The rate constant when the initial [ NC] is 0.030 mol L<sup>-1</sup> is ________ .](https://storage.examlex.com/TB5343/11ea7a5e_3e80_e3c5_89bd_d939c18ec01b_TB5343_11.jpg) NC(g)→
NC(g)→ ![The isomerization of methylisonitrile to acetonitrile NC(g)→ CN(g) Is first order in NC.The half-life of the reaction is 5.20 × 10<sup>1</sup> s at 545 K.The rate constant when the initial [ NC] is 0.030 mol L<sup>-1</sup> is ________ .](https://storage.examlex.com/TB5343/11ea7a5e_3e80_e3c6_89bd_6f455242c774_TB5343_11.jpg) CN(g)
Is first order in
CN(g)
Is first order in ![The isomerization of methylisonitrile to acetonitrile NC(g)→ CN(g) Is first order in NC.The half-life of the reaction is 5.20 × 10<sup>1</sup> s at 545 K.The rate constant when the initial [ NC] is 0.030 mol L<sup>-1</sup> is ________ .](https://storage.examlex.com/TB5343/11ea7a5e_3e80_e3c7_89bd_555132ab2a5b_TB5343_11.jpg) NC.The half-life of the reaction is 5.20 × 101 s at 545 K.The rate constant when the initial [
NC.The half-life of the reaction is 5.20 × 101 s at 545 K.The rate constant when the initial [ ![The isomerization of methylisonitrile to acetonitrile NC(g)→ CN(g) Is first order in NC.The half-life of the reaction is 5.20 × 10<sup>1</sup> s at 545 K.The rate constant when the initial [ NC] is 0.030 mol L<sup>-1</sup> is ________ .](https://storage.examlex.com/TB5343/11ea7a5e_3e81_0ad8_89bd_0dc784ff8e03_TB5343_11.jpg) NC] is 0.030 mol L-1 is ________
NC] is 0.030 mol L-1 is ________ ![The isomerization of methylisonitrile to acetonitrile NC(g)→ CN(g) Is first order in NC.The half-life of the reaction is 5.20 × 10<sup>1</sup> s at 545 K.The rate constant when the initial [ NC] is 0.030 mol L<sup>-1</sup> is ________ .](https://storage.examlex.com/TB5343/11ea7a5e_3e81_0ad9_89bd_c303b11fbc1b_TB5343_11.jpg) .
.
(Multiple Choice)
4.7/5  (44)
(44)
The rate constant at 160 °C for the first order decomposition of ore is 0.032/min.The half-life of the reaction is ________.
(Multiple Choice)
4.8/5  (39)
(39)
For the second-order reaction A → products,the following data are obtained:
[A] = 3.024 M,t = 0 min
[A] = 2.935 M,t = 1.0 min
[A] = 2.852 M,t = 2.0 min
What is the concentration of [A] after 4 min?
(Multiple Choice)
4.9/5  (38)
(38)
Given the following initial rate data,write the rate law expression. 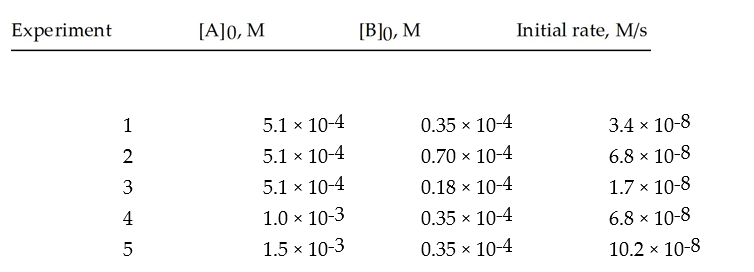
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)