Exam 17: Additional Aspects of Acidbase Equilibria
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What mass of sodium acetate should be dissolved in 250.0 mL of 0.30 M aqueous acetic acid to form a buffer of pH 5.0? (Ka for acetic acid is 1.8 × 10-5)
Free
(Multiple Choice)
5.0/5  (35)
(35)
Correct Answer:
A
What is the pH of a buffer system prepared by dissolving 10.70 grams of NH4Cl and 25.00 mL of 12 mol L-1 NH3 in enough water to make 1.000 L of solution?
Kb = 1.80 × 10-5 for NH3.
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
25 mL of 0.10 M aqueous acetic acid is titrated with 0.10 M NaOH(aq).What is the pH at the equivalence point? Ka for acetic acid = 1.8 × 10-5.
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
D
In the titration of 50.0 mL of 0.0200 M C6H5COOH(aq)with 0.100 M NaOH(aq),what is/are the major species in the solution after the addition of 5.0 mL of NaOH(aq)?
(Multiple Choice)
4.8/5  (43)
(43)
What is the pH of the resulting solution if 45 mL of 0.432 mol L-1 aqueous methylamine,CH3NH2,is added to 15 mL of 0.234 mol L-1 HCl(aq)?
Assume that the volumes of the solutions are additive.Ka = 2.70 × 10-11 for CH3NH3+.
(Multiple Choice)
4.9/5  (27)
(27)
Calculate the pH of a 1.00 L solution of 0.100 M NH3(aq)after the addition of 0.010 mol NH4Cl(s).For NH3,pKb = 4.74.
(Multiple Choice)
4.9/5  (30)
(30)
If 25 mL of 0.20 M NaOH(aq)is added to 50 mL of 0.10 M aqueous HC2H3O2 (Ka = 1.8 × 10-5),what is the pH?
(Multiple Choice)
4.8/5  (40)
(40)
An acid has a Ka = 1 × 10-6.At what pH would this acid and its corresponding salt make a good buffer?
(Multiple Choice)
4.8/5  (33)
(33)
What is the pH of an aqueous solution prepared by mixing 50.00 mL of 0.10 mol L-1 NH3 with 5.00 mL of 0.10 mol L-1 NH4Cl?
Assume that the volume of the solutions are additive and that Kb = 1.8 × 10-5 for NH3.
(Multiple Choice)
4.8/5  (38)
(38)
What is the [CH3COO-]/[CH3COOH] ratio necessary to make an aqueous buffer solution with a pH of 4.34?
Ka = 1.8 × 10-5 for CH3COOH.
(Multiple Choice)
4.8/5  (30)
(30)
Which among the following pairs is inefficient as buffer pair?
(Multiple Choice)
4.9/5  (36)
(36)
Twenty-five milliliters of 0.10 M HCl(aq)is titrated with 0.10 M NaOH(aq).What is the pH when 30 mL of NaOH(aq)have been added?
(Multiple Choice)
4.8/5  (26)
(26)
Calculate the pH of an aqueous solution that is 0.210 mol L-1 in nitrous acid ( 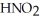 ) and 0.290 mol L-1 in potassium nitrite (
) and 0.290 mol L-1 in potassium nitrite ( 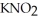 ).The acid dissociation constant of nitrous acid is 4.50 ×
).The acid dissociation constant of nitrous acid is 4.50 × 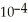 .
.
(Multiple Choice)
4.9/5  (30)
(30)
Determine the [F-] of the following aqueous solution.Initial concentrations are given.
[HF] = 1.296 M,[NaF] = 1.045 M,Ka for HF is 6.6 × 10-4
(Multiple Choice)
4.8/5  (32)
(32)
The pH of a buffer solution changes only slightly with addition of a small amount of acid or base.
(True/False)
5.0/5  (36)
(36)
How will addition of sodium chloride affect the pH of a HCl(aq)solution?
(Multiple Choice)
4.9/5  (32)
(32)
What is the pH of a 0.30 M aqueous trisodium phosphate solution? Ka for monohydrogen phosphate ion is 4.2 × 10-13
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements correctly describe a typical titration curve for the titration of a strong acid by a strong base?
I.The beginning pH is low.
II.The pH change is slow until near the equivalence point.
III.At the equivalence point,pH changes by a large value.
IV.Beyond the equivalence point,pH rises rapidly.
V.The equivalence point would be at a pH less than 3.5.
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the pH of an aqueous solution that is 1.0 M Na2CO3.Ka2 for H2CO3 is 4.7 × 10-11.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)