Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Using the VSEPR model,the molecular geometry of the central atom in H2Se is:
Free
(Multiple Choice)
5.0/5  (34)
(34)
Correct Answer:
D
Which of the following statements concerning the carbon dioxide molecule is correct?
(Multiple Choice)
4.7/5  (35)
(35)
The valence-bond method describes covalent bonding as the overlap of partially filled orbitals.
(True/False)
4.9/5  (36)
(36)
Which of the following carbon molecules has sp hybridization?
(Multiple Choice)
5.0/5  (32)
(32)
One resonance structure of N2O is 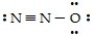 The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
(Multiple Choice)
4.9/5  (40)
(40)
Using the VSEPR model,the molecular geometry of the central atom in NCl3 is:
(Multiple Choice)
5.0/5  (43)
(43)
The valence-bond method provides energy information about molecules.
(True/False)
4.9/5  (38)
(38)
The structure of aspirin is given below.(Note that lone pairs are not shown. ) 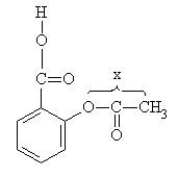 What is the O-C-C bond angle,"x," in aspirin?
What is the O-C-C bond angle,"x," in aspirin?
(Multiple Choice)
4.9/5  (38)
(38)
According to the principles of VSEPR applied on ICl5,which of the following is INCORRECT?
(Multiple Choice)
4.7/5  (42)
(42)
Using the VSEPR model,the electron-group geometry of the central atom in SF4 is:
(Multiple Choice)
4.9/5  (27)
(27)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)