Exam 18: Solubility and Complex-Ion Equilibria
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
To a saturated aqueous solution of barium carbonate is added just enough sodium sulfate to achieve a maximum sulfate concentration without precipitation of barium sulfate.If no complexes form and the "salt effect" is negligible,what is the concentration of the sulfate ion in this solution? [Ksp for barium carbonate is 1.6 × 10-9;Ksp for barium sulfate is 7.9 × 10-11]
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Calculate the molar solubility of thallium chloride (TlCl)in 0.30 mol L-1 NaCl(aq)at 25 °C.Ksp for TlCl(s)is 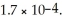
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
B
Which of the following is least soluble?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
To a concentrated buffer of pH 9.0 was added an equal volume of an aqueous solution that was 0.20 M in each of the ions Ca2+,Cd2+,and Cu2+.The expected precipitate would consist of:
Salt: calcium hydroxide cadmium hydroxide copper(II)hydroxide
Ksp: 4.0 × 10-6 2.0 × 10-14 1.8 × 10-19
(Multiple Choice)
4.7/5  (34)
(34)
What is the concentration of free Zn2+(aq)if 0.020 M Zn2+ solution is mixed with an equal volume of 2.0 M NH3(aq)? Kf for [Zn(NH3)4]2+ is 4.1 × 108.
(Multiple Choice)
4.8/5  (34)
(34)
The following table lists five compounds and their Ksp value.Which is least soluble?

(Multiple Choice)
5.0/5  (35)
(35)
Which of the following should dissolve the smallest amount of silver sulfide per liter,assuming no complex formation?
(Multiple Choice)
4.8/5  (37)
(37)
In a qualitative cation analysis,the unknown ion is not precipitated by aqueous solutions of HCl(aq),H2S(aq),or CO32-(aq).A flame test produced a violet flame.The unknown ion is ________.
(Multiple Choice)
4.9/5  (26)
(26)
What is the free Cu2+(aq)concentration if 0.020 M Cu2+(aq)solution is mixed with an equal volume of 4.0 M NH3(aq)? Kf for [Cu(NH3)4]2+ is 1.1 × 1013.
(Multiple Choice)
4.8/5  (28)
(28)
The solubility of cerium iodate,Ce(IO3)3,molar mass = 664.83 g/mol,in pure water is 124 mg per 100 mL of water.Calculate the solubility product constant for cerium iodate.
(Multiple Choice)
4.8/5  (38)
(38)
An aqueous solution contains [Ba2+] = 5.0 × 10-5 M,[Ag+] = 3.0 × 10-5 M,and [Zn2+] = 2.0 × 10-7 M.Sodium oxalate is slowly added so that [C2O42-] increases.
![An aqueous solution contains [Ba<sup>2+</sup>] = 5.0 × 10<sup>-5</sup> M,[Ag<sup>+</sup>] = 3.0 × 10<sup>-5</sup> M,and [Zn<sup>2+</sup>] = 2.0 × 10<sup>-7</sup> M.Sodium oxalate is slowly added so that [C<sub>2</sub>O<sub>4</sub><sup>2-</sup>] increases. Which one of the oxalates precipitates first?](https://storage.examlex.com/TB5343/11ec76b4_fecf_93ba_9e6f_d7386ce87ce1_TB5343_00.jpg) Which one of the oxalates precipitates first?
Which one of the oxalates precipitates first?
(Multiple Choice)
4.9/5  (37)
(37)
Concentrated aqueous solutions of iron (III)chloride,sodium hydroxide,and potassium nitrate are mixed together.The precipitate which forms is ________.
(Multiple Choice)
4.9/5  (37)
(37)
What is the molar solubility of barium fluoride ( BaF2 )in water? The solubility-product constant for BaF2 (s)is 1.7 × 10-6 at 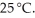
(Multiple Choice)
4.8/5  (36)
(36)
When 100 mL each of 2.0 × 10-5 M Ca2+(aq)and 2.0 × 10-3 M CO32-(aq)are mixed,what is the remaining Ca2+(aq)ion concentration and is precipitation complete? The solubility product constant of CaCO3(s)is 2.8 × 10-9.
(Multiple Choice)
4.8/5  (38)
(38)
Qualitative cation analysis has been replaced in recent years by instrumental analysis.
(True/False)
4.8/5  (33)
(33)
What is the molar solubility of AgCl in 0.40 mol L-1 NH3(aq)? Ksp for AgCl(s)is 1.8 × 10-10 and Kf for Ag(NH3)2+ is 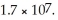
(Multiple Choice)
4.8/5  (38)
(38)
The solubility product constant of Li3PO4 is 3.2 × 10-9.What is the molar solubility of Li3PO4 in water?
(Multiple Choice)
4.7/5  (38)
(38)
Consider an aqueous solution which is 0.020 M in Pb2+ and 0.20 M in Ag+.If concentrated aqueous potassium iodide solution (so that volume changes may be neglected)is added gradually with good stirring to this solution,which precipitate will form first?
Ksp for PbI2 = 7.1 × 10-9;for AgI = 8.31 × 10-17
(Multiple Choice)
4.9/5  (46)
(46)
Calculate the Ksp for silver carbonate if the solubility of Ag2CO3(s)in pure water is 3.5 × 10-2 g L-1.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)