Exam 27: Reactions of Organic Compounds
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Which of the following alcohols can eliminate water to produce alkene?
I.phenol
II.HOC(CH3)3
III.HOCH(CH3)2
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
Give the product(s)of the reaction (in H2SO4):
CH2=CHCH3 + H2O → product(s)
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
E
The step reaction polymerizations are fast polymerization reactions.
Free
(True/False)
4.9/5  (32)
(32)
Correct Answer:
False
Reactions between alkanes and HX compounds are typical examples of addition reactions.
(True/False)
4.8/5  (42)
(42)
A hydrocarbon has a molecular weight 56 g/mol and contains 85.7% carbon.It easily adds one mol of HBr to produce a chiral alkyl bromide.Which of the following structures could be the hydrocarbon?
(Multiple Choice)
4.8/5  (32)
(32)
You need to substitute one hydrogen with bromine on an aromatic ring.What reagents do you use?
(Multiple Choice)
4.7/5  (39)
(39)
Suggest reagents for the synthesis of meta-chloronitrobenzene from nitrobenzene.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements correctly describe SN2 and SN1 reactions?
I.SN2 reactions proceed with retention of configuration.
II.SN1 reactions prefer polar protic solvents.
III.SN1 reactions can produce racemic products.
IV.SN2 reactions are promoted in the presence of a substrate that produces a very stable carbocation.
V.SN1 reactions have a unimolecular rate-determining step.
(Multiple Choice)
4.9/5  (37)
(37)
Classify the following polymers as atactic,isotactic or syndiotactic: 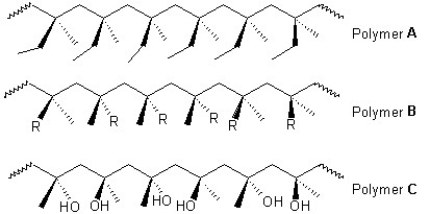
(Multiple Choice)
4.9/5  (35)
(35)
Markovnikof's rule predicts that when HBr is added to an unsymmetrical alkene or alkyne,the H atom will add to which carbon atom?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following compounds could be used as catalysts in halogenation of benzene?
I.AlCl3
II.NaCl
III.FeBr3
IV.MgCl2
V.PbCl2
(Multiple Choice)
4.9/5  (50)
(50)
Suggest possible compounds for A,B and C in the following scheme: 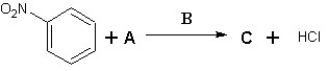
(Multiple Choice)
4.8/5  (32)
(32)
What is the major product in the reaction between 2-bromo-3-methylbutane and KOtBu in ethanol?
(Multiple Choice)
4.7/5  (38)
(38)
Molecules add across multiple bonds in another molecule in addition reactions.
(True/False)
4.8/5  (28)
(28)
Which of the following steps are a part of chain reaction during alkane substitution?
I.propagation
II.initiation
III.competition
IV.dehalogenation
V.termination
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)