Exam 3: Chemical Reactions and Reaction Stoichiometry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
When the following equation is balanced,the coefficients are ________. 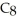
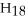 + O2 → CO2 + H2O
+ O2 → CO2 + H2O
(Multiple Choice)
4.7/5  (31)
(31)
A nitrogen oxide is 63.65% by mass nitrogen.The molecular formula could be ________.
(Multiple Choice)
4.7/5  (36)
(36)
Combustion of a 0.9827-g sample of a compound containing only carbon,hydrogen,and oxygen produced 1.900 g of CO2 and 1.070 g of H2O.What is the empirical formula of the compound?
(Multiple Choice)
5.0/5  (30)
(30)
The combustion of propane (C3H8)in the presence of excess oxygen yields CO2 and H2O:
C3H8 (g)+ 5O2 (g)→ 3CO2 (g)+ 4H2O (g)
How many grams of CO2 is produced when 12.5 g of C3H8 burns in open air?
(Short Answer)
4.8/5  (26)
(26)
What is the empirical formula of a compound that contains 49.4% K,20.3% S,and 30.3% O by mass?
(Multiple Choice)
4.7/5  (28)
(28)
A certain alcohol contains only three elements,carbon,hydrogen,and oxygen.Combustion of a 60.00 gram sample of the alcohol produced 114.6 grams of CO2 and 70.44 grams of H2O.What is the empirical formula of the alcohol?
(Short Answer)
4.9/5  (42)
(42)
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: 4 NH3 (g)+ 7 O2 (g)→ 4 NO2 (g)+ 6 H2O (g)
The combustion of 14.4 g of ammonia consumes ________ g of oxygen.
(Multiple Choice)
4.9/5  (34)
(34)
When the following equation is balanced,the coefficient of HNO3 is ________. HNO3 (aq)+ CaCO3 (s)→ Ca(NO3)2 (aq)+ CO2 (g)+ H2O (l)
(Multiple Choice)
4.8/5  (34)
(34)
The molecular formula of aspartame,the generic name of NutraSweet®,is C14H18N2O5.What is the mass (g)of 1.00 mol of aspartame?
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the percentage by mass of hydrogen in PtCl2(NH3)2.
(Multiple Choice)
4.7/5  (28)
(28)
GeF3H is formed from GeH4 and GeF4 in the combination reaction: GeH4 + 3GeF4 → 4GeF3H
If the reaction yield is 89.1%,how many moles of GeH4 are needed to produce 3.50 mol of GeF3H?
(Multiple Choice)
4.8/5  (32)
(32)
A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass.What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (47)
(47)
1 mole of which of the following will have the largest mass?
(Multiple Choice)
4.7/5  (38)
(38)
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li (s)+ N2 (g)→ 2Li3N (s)
In a particular experiment,2.50-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
(Multiple Choice)
4.9/5  (44)
(44)
When the following equation is balanced,the coefficients are ________. Al(NO3)3 + Na2S → Al2S3 + NaNO3
(Multiple Choice)
4.9/5  (39)
(39)
Lead (II)carbonate decomposes to give lead (II)oxide and carbon dioxide: PbCO3 (s)→ PbO (s)+ CO2 (g)
________ grams of lead (II)oxide will be produced by the decomposition of 8.75 g of lead (II)carbonate?
(Multiple Choice)
4.7/5  (32)
(32)
A 30.5 gram sample of glucose (C6H12O6)contains ________ mol of glucose.
(Multiple Choice)
4.8/5  (30)
(30)
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li (s)+ N2 (g)→ 2Li3N (s)
How many moles of lithium are needed to produce 0.45 mol of Li3N when the reaction is carried out in the presence of excess nitrogen?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 121 - 140 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)