Exam 3: Chemical Reactions and Reaction Stoichiometry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 2NaN3 (s)→ 2Na (s)+ 3N2 (g)
How many moles of N2 are produced by the decomposition of 1.75 mol of sodium azide?
(Multiple Choice)
4.8/5  (35)
(35)
When the following equation is balanced,the coefficient of O2 is ________. C2H4O (g)+ O2 (g)→ CO2 (g)+ H2O (g)
(Multiple Choice)
4.7/5  (40)
(40)
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s)+ H2O (l)→ Ca(OH)2 (s)
A 3.50 g sample of CaO is reacted with 3.38 g of H2O.How many grams of water remain after completion of reaction?
(Multiple Choice)
4.9/5  (35)
(35)
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 Mg + N2 → Mg3N2
In a particular experiment,a 8.33-g sample of 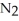 reacts completely.The mass of Mg consumed is ________ g.
reacts completely.The mass of Mg consumed is ________ g.
(Multiple Choice)
4.8/5  (33)
(33)
When the following equation is balanced,the coefficient of dinitrogen pentoxide is ________. N2O5 (g)+ H2O (l)→ HNO3 (aq)
(Multiple Choice)
4.9/5  (43)
(43)
A sulfur oxide is 50.0% by mass sulfur.This molecular formula could be ________.
(Multiple Choice)
4.9/5  (26)
(26)
The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide.The combustion of 10 mol of CS2 in the presence of excess oxygen yields ________ mol of SO2.
(Short Answer)
4.8/5  (32)
(32)
Water can be formed from the stoichiometric reaction of hydrogen with oxygen.How many grams of H2O is generated when 45.3 g of O2 reacts with excess hydrogen?
(Short Answer)
4.8/5  (43)
(43)
Balance the following reaction and determine the coefficient of nitric acid. N2O5 (g)+ H2O (l)→ HNO3 (aq)
(Multiple Choice)
4.8/5  (36)
(36)
A compound that is composed of only carbon and hydrogen contains 80.0% C and 20.0% H by mass.What is the empirical formula of the compound?
(Multiple Choice)
5.0/5  (33)
(33)
The formula weight of potassium dichromate (K2Cr2O7)is ________ amu.
(Multiple Choice)
5.0/5  (29)
(29)
What mass in grams of hydrogen is produced by the reaction of 31.3 g of magnesium with 2.12 g of water?
Mg (s)+ 2H2O (l)→ Mg(OH)2 (s)+ H2 (g)
(Multiple Choice)
4.8/5  (32)
(32)
Under appropriate conditions,nitrogen and hydrogen undergo a combination reaction to yield ammonia: N2 (g)+ 3H2 (g)→ 2NH3 (g)
If the reaction yield is 81.4 %,how many moles of N2 are needed to produce 8.50 mol of NH3?
(Multiple Choice)
4.8/5  (31)
(31)
The combustion of ammonia in the presence of oxygen yields NO2 and H2O: 4 NH3 (g)+ 7 O2 (g)→ 4 NO2 (g)+ 6 H2O (g)
The combustion of 43.9 g of ammonia with 258 g of oxygen produces ________ g of NO2.
(Multiple Choice)
4.8/5  (26)
(26)
Sulfur and oxygen react to produce sulfur trioxide.In a particular experiment,7.9 grams of SO3 are produced by the reaction of 6.0 grams of O2 with 7.0 grams of S.What is the % yield of SO3 in this experiment?
S (s)+ O2 (g)→ SO3 (g)(not balanced)
(Multiple Choice)
4.8/5  (39)
(39)
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide,an environmental pollutant: 2S (s)+ 3O2 (g)→ 2SO3 (g)
In a particular experiment,the reaction of 1.0 g S with 1.0 g O2 produced 0.80 g of SO3.The % yield in this experiment is ________.
(Multiple Choice)
4.8/5  (38)
(38)
What is the mass in grams of 9.76 × 1012 atoms of naturally occurring potassium?
(Multiple Choice)
4.7/5  (39)
(39)
The formula weight of calcium nitrate (Ca(NO3)2),rounded to one decimal place,is ________ amu.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 141 - 160 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)