Exam 3: Chemical Reactions and Reaction Stoichiometry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Calcium carbide (CaC2)reacts with water to produce acetylene (C2H2): CaC2 (s)+ 2H2O (g)→ Ca(OH)2 (s)+ C2H2 (g)
Production of 13 g of C2H2 requires consumption of ________ g of H2O.
(Multiple Choice)
4.9/5  (34)
(34)
Which one of the following substances is the product of this combination reaction?
Al (s)+ I2(s)→ ________
(Multiple Choice)
4.9/5  (29)
(29)
The combustion of propane (C3H8)produces CO2 and H2O: C3H8 (g)+ 5O2 (g)→ 3CO2 (g)+ 4H2O (g)
The reaction of 2.5 mol of O2 with 4.6 mol of C3H8 will produce ________ mol of H2O.
(Multiple Choice)
4.7/5  (32)
(32)
What is the mass % of carbon in dimethylsulfoxide (C2H6SO)rounded to three significant figures?
(Multiple Choice)
4.8/5  (27)
(27)
The combustion of propane (C3H8)produces CO2 and H2O: C3H8 (g)+ 5O2 (g)→ 3CO2 (g)+ 4H2O (g)
The reaction of 2.5 mol of O2 will produce ________ mol of H2O.
(Multiple Choice)
4.9/5  (38)
(38)
When the following equation is balanced,the coefficients are ________. C8H18 + O2 → CO2 + H2O
(Multiple Choice)
4.7/5  (31)
(31)
Combustion of a 1.031-g sample of a compound containing only carbon,hydrogen,and oxygen produced 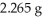 of CO2 and
of CO2 and 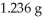 of H2O.What is the empirical formula of the compound?
of H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.7/5  (28)
(28)
What is the maximum mass in grams of NH3 that can be produced by the reaction of 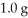 of N2 with
of N2 with 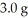 of H2 via the equation below?
N2 (g)+ H2 (g)→ NH3 (g)(not balanced)
of H2 via the equation below?
N2 (g)+ H2 (g)→ NH3 (g)(not balanced)
(Multiple Choice)
4.7/5  (35)
(35)
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: S (s)+ 3F2(g)→ SF6 (g)
The maximum amount of SF6 that can be produced from the reaction of 32.1 g of sulfur with 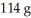 of fluorine is ________ g.
of fluorine is ________ g.
(Multiple Choice)
4.9/5  (33)
(33)
Of the reactions below,which one is a decomposition reaction?
(Multiple Choice)
4.9/5  (28)
(28)
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: 4 NH3 (g)+ 7 O2 (g)→ 4 NO2 (g)+ 6 H2O (g)
The combustion of 43.9 g of ammonia produces ________ g of NO2.
(Multiple Choice)
4.8/5  (35)
(35)
When the following equation is balanced,the coefficient of Al2O3 is ________. Al2O3 (s)+ C (s)+ Cl2 (g)→ AlCl3 (s)+ CO (g)
(Multiple Choice)
4.9/5  (32)
(32)
What is the empirical formula of a compound that contains 29% Na,41% S,and 30% O by mass?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 21 - 40 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)