Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which of these metals will be oxidized by the ions of aluminum?
(Multiple Choice)
4.8/5  (45)
(45)
Zinc is more active than cobalt and iron but less active than aluminum.Cobalt is more active than nickel but less active than iron.Which of the following correctly lists the elements in order of increasing activity?
(Multiple Choice)
4.8/5  (33)
(33)
What mass (g)of barium iodide is contained in 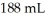 of a barium iodide solution that has an iodide ion concentration of
of a barium iodide solution that has an iodide ion concentration of 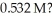
(Multiple Choice)
4.8/5  (37)
(37)
Which of these metals is the most easily oxidized?
Cu
Au
Pt
Li
Hg
(Multiple Choice)
4.9/5  (36)
(36)
325 mL of a 5.50 M phosphoric acid solution was prepared for laboratory.How many grams of phosphoric acid was in this solution.
(Short Answer)
4.9/5  (31)
(31)
The balanced molecular equation for complete neutralization of H2SO4 by KOH in aqueous solution is ________.
(Multiple Choice)
4.8/5  (44)
(44)
In which species does nitrogen have an oxidation number of zero?
(Multiple Choice)
4.8/5  (47)
(47)
The spectator ions in the reaction between aqueous hydrobromic acid and aqueous sodium hydroxide are ________.
(Multiple Choice)
4.7/5  (26)
(26)
How many moles of chloride ions are present in 0.300 L of a 0.150 M solution of AlCl3?
(Short Answer)
4.9/5  (36)
(36)
The concentration (M)of an aqueous methanol produced when 0.200 L of a 2.00 M solution was diluted to 0.800 L is ________.
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following is a correct expression for molarity?
(Multiple Choice)
4.8/5  (39)
(39)
A solution of CH3F is prepared by dissolving 17.2 g of CH3F in sufficient water to give exactly 300 mL of solution.What is the concentration (M)of the newly prepared CH3F solution?
(Short Answer)
4.7/5  (37)
(37)
Mixing 10.00 mL of an aqueous solution with 10.00 mL of water represents a ________.
(Multiple Choice)
4.9/5  (45)
(45)
How many moles of Co2+ are present in 0.200 L of a 0.400 M solution of CoI2?
(Multiple Choice)
4.9/5  (42)
(42)
A compound was found to be soluble in water. It was also found that addition of acid to an aqueous solution of this compound resulted in the formation of carbon dioxide.Which one of the following cations would form a precipitate when added to an aqueous solution of this compound?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 41 - 60 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)