Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
How many grams of NaOH (MW = 40.0)are there in 200.0 mL of a 0.175 M NaOH solution?
(Multiple Choice)
4.9/5  (46)
(46)
Based on the equations below,which metal is the most active?
Pb(NO3)2 (aq)+ Ni (s)→ Ni(NO3)2 (aq)+ Pb (s)
Pb(NO3)2 (aq)+ Ag (s)→ No reaction
Cu(NO3)2 (aq)+ Ni (s)→ Ni(NO3)2 (aq)+ Cu (s)
(Multiple Choice)
5.0/5  (40)
(40)
How many milliliters of 1.500 M aqueous KI solution must be added to an aqueous solution containing 0.100 mol of Pb(NO3)2 to completely precipitate the lead?
(Multiple Choice)
4.8/5  (27)
(27)
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is ________.
(Multiple Choice)
4.8/5  (37)
(37)
The net ionic equation for the reaction between aqueous nitric acid and aqueous sodium hydroxide is ________.
(Multiple Choice)
4.7/5  (30)
(30)
A 650 mL sodium bromide solution has a bromide ion concentration of 0.245 M.What is the mass (g)of sodium bromide in solution?
(Multiple Choice)
4.9/5  (34)
(34)
How many milliliters of a 14.2 M HCl solution is needed to prepare 0.715 L solution of HCl with a concentration of 0.350 M?
(Multiple Choice)
4.7/5  (27)
(27)
The molarity of a solution prepared by diluting 43.72 mL of 5.005 M aqueous K2Cr2O7 to 500.mL is ________.
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following would require the largest volume of 0.100 M sodium hydroxide solution for neutralization?
(Multiple Choice)
4.9/5  (30)
(30)
What volume (mL)of a concentrated solution of sodium hydroxide (6.00 M)must be diluted to 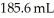 to make a
to make a 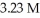 solution of sodium hydroxide?
solution of sodium hydroxide?
(Multiple Choice)
4.9/5  (34)
(34)
The point in a titration at which the indicator changes is called the ________.
(Multiple Choice)
4.9/5  (30)
(30)
The process by which metal in the presence of air and water is converted into rust is known as ________.
(Multiple Choice)
4.9/5  (38)
(38)
The spectator ions in the reaction between aqueous hydrobromic acid and aqueous ammonia are ________.
(Multiple Choice)
4.8/5  (35)
(35)
When 0.344 mol of HCl is combined with enough water to make a 450.0 mL solution,the concentration of HCl is ________ M.
(Multiple Choice)
4.8/5  (31)
(31)
What is the concentration (M)of CH3OH in a solution prepared by dissolving 34.4 g of CH3OH in sufficient water to give exactly 230 mL of solution?
(Multiple Choice)
4.9/5  (39)
(39)
With which of the following will the Potassium ion form an insoluble salt?
(Multiple Choice)
4.9/5  (37)
(37)
With which of the following will the ammonium ion form an insoluble salt?
(Multiple Choice)
5.0/5  (33)
(33)
Showing 81 - 100 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)