Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which one of the following solutions will have the greatest concentration of hydroxide ions?
(Multiple Choice)
4.8/5  (34)
(34)
What volume (L)of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following compounds is insoluble in water?
(Multiple Choice)
4.7/5  (32)
(32)
The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is ________.
(Multiple Choice)
4.9/5  (32)
(32)
The net ionic equation for the reaction between aqueous solutions of HF and KOH is ________.
(Multiple Choice)
4.8/5  (36)
(36)
In which reaction does the oxidation number of oxygen increase?
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the concentration (M)of sodium ions in a solution made by diluting 50.0 mL of a 0.874 M solution of sodium sulfide to a total volume of 250.0 mL.
(Multiple Choice)
4.8/5  (37)
(37)
The molarity (M)of an aqueous solution containing 129 g of glucose (C6H12O6)in 200 mL of solution is ________.
(Multiple Choice)
4.8/5  (29)
(29)
What mass (g)of AgBr is formed when 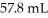 of
of 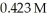 AgNO3 is treated with an excess of aqueous hydrobromic acid?
AgNO3 is treated with an excess of aqueous hydrobromic acid?
(Multiple Choice)
4.7/5  (28)
(28)
A 25.5 mL aliquot of HCl (aq)of unknown concentration was titrated with 0.113 M NaOH (aq).It took 51.2 mL of the base to reach the endpoint of the titration.The concentration (M)of the acid was ________.
(Multiple Choice)
4.8/5  (29)
(29)
When aqueous solutions of AgNO3 and KI are mixed,silver iodide precipitates.The balanced net ionic equation is ________.
(Multiple Choice)
4.8/5  (38)
(38)
What volume (mL)of 0.102 M NaOH is required to neutralize 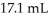 of
of 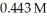 HCl?
HCl?
(Multiple Choice)
4.8/5  (36)
(36)
A 31.5 mL aliquot of H2SO4 (aq)of unknown concentration was titrated with 0.0134 M NaOH (aq).It took 23.9 mL of the base to reach the endpoint of the titration.The concentration (M)of the acid was ________.
(Multiple Choice)
4.7/5  (38)
(38)
If an unknown sample contains 39.04% sulfuric acid by mass,then a 0.9368 g of that sample would require ________ mL of 0.2389 M NaOH for neutralization.
(Multiple Choice)
4.9/5  (38)
(38)
What is the concentration (M)of potassium ions in 153 mL of a 1.25 M K3PO4 solution?
(Short Answer)
4.8/5  (38)
(38)
The molarity of a solution prepared by diluting 43.72 mL of 1.005 M aqueous K2Cr2O7 to 500.mL is ________.
(Multiple Choice)
4.7/5  (38)
(38)
An aqueous ethanol solution (400 mL)was diluted to 4.00 L,giving a concentration of 0.0400 M.The concentration of the original solution was ________ M.
(Multiple Choice)
4.8/5  (33)
(33)
Of the choices below,which would be the best for the lining of a tank intended for use in storage of hydrochloric acid?
(Multiple Choice)
4.7/5  (26)
(26)
Showing 61 - 80 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)