Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
A weak electrolyte exists predominantly as ________ in solution.
(Multiple Choice)
4.9/5  (42)
(42)
How much CaF2 (g)is formed when 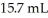 of
of 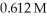 KF reacts with an excess of aqueous calcium bicarbonate?
KF reacts with an excess of aqueous calcium bicarbonate?
(Multiple Choice)
4.8/5  (31)
(31)
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: Ag+ (aq)+ Cl- (aq)→ AgCl (s)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.149 M AgNO3 solution to completely precipitate the silver?
(Multiple Choice)
4.8/5  (35)
(35)
What is the concentration (M)of potassium ions in a solution made by diluting 40.0 mL of a 0.474 M solution of potassium sulfide to a total volume of 300 mL.
(Short Answer)
4.9/5  (35)
(35)
Based on the activity series,which one of the reactions below will occur?
(Multiple Choice)
4.9/5  (37)
(37)
How many grams of CH3OH must be added to water to prepare 150 mL of a solution that is 2.0 M CH3OH?
(Multiple Choice)
4.8/5  (36)
(36)
What is the concentration of chloride ions in a 0.349 M solution of sodium chloride?
(Multiple Choice)
4.8/5  (39)
(39)
Of the following elements,________ is the most difficult to oxidize. Cu
Hg
Au
Ag
Na
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following are strong electrolytes?
HCl
HC2H3O2
NH3
KCl
(Multiple Choice)
4.9/5  (40)
(40)
In which species does bromine have an oxidation number of zero?
(Multiple Choice)
4.8/5  (37)
(37)
0.400 g of solid NaCl is added to 50 mL of a 0.100 M MgCl2 solution.Assuming the final volume does not change,what is the molarity of chloride ion in the final solution?
(Multiple Choice)
4.9/5  (32)
(32)
Of the metals below,which will dissolve in an aqueous solution containing nickel ion?
Aluminum
Chromium
Barium
Tin
Potassium
(Multiple Choice)
4.8/5  (31)
(31)
What are the respective concentrations (M)of K+ and CO32- afforded by dissolving 0.530 mol K2CO3 in water and diluting to 1.50 L?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the number of grams of unknown solute (MW = 56.105 g/mol)in 250.0 mL of a 0.169 M solution.
(Multiple Choice)
4.8/5  (39)
(39)
What is the concentration (M)of a NaCl solution prepared by dissolving 9.3 g of NaCl in sufficient water to give 350 mL of solution?
(Multiple Choice)
4.7/5  (30)
(30)
All of the following are true concerning 2.00 L of 0.100 M solution of Ca3(PO4)2 except for ________.
(Multiple Choice)
4.7/5  (38)
(38)
The molarity (M)of an aqueous solution containing 85.1 g of sucrose (C12H22O11)in 128 mL of solution is ________.
(Multiple Choice)
4.8/5  (41)
(41)
What is the concentration of nitrate ions in a 0.343 M solution of lead (II)nitrate?
(Multiple Choice)
4.9/5  (33)
(33)
The molarity (M)of an aqueous solution containing 22.5 g of sucrose (C12H22O11)in 35.5 mL of solution is ________.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 141 - 160 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)