Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
What are the respective concentrations (M)of Fe3+ and I- afforded by dissolving 0.200 mol FeI3 in water and diluting to 725 mL?
(Multiple Choice)
4.8/5  (30)
(30)
The spectator ions in the reaction between aqueous perchloric acid and aqueous barium hydroxide are ________.
(Multiple Choice)
4.8/5  (27)
(27)
The concentration of iodide ions in a 0.193 M solution of barium iodide is ________.
(Multiple Choice)
4.8/5  (35)
(35)
How many grams of NaOH (MW = 40.0)are there in 500.0 mL of a 0.250 M NaOH solution?
(Multiple Choice)
4.8/5  (26)
(26)
What is the concentration (M)of KCl in a solution made by mixing 25.0 mL of 0.100 M KCl with 50.0 mL of 0.100 M KCl?
(Multiple Choice)
4.9/5  (32)
(32)
The total concentration of ions in a 0.250 M solution of HCl is ________.
(Multiple Choice)
4.9/5  (39)
(39)
A strong electrolyte is one that ________ completely in solution.
(Multiple Choice)
4.9/5  (49)
(49)
What are the spectator ions in the reaction between Mg(OH)2 (aq)and HCl (aq)?
(Multiple Choice)
4.9/5  (34)
(34)
Which one of the following substances is produced during the reaction of an acid with a metal hydroxide?
(Multiple Choice)
4.8/5  (32)
(32)
How many moles of chloride ions are in 0.500 L of a 0.250 M solution of AlCl3?
(Multiple Choice)
4.8/5  (33)
(33)
The net ionic equation for formation of an aqueous solution of Al(NO3)3 via mixing solid Al(OH)3 and aqueous nitric acid is ________.
(Multiple Choice)
4.9/5  (32)
(32)
If it took 49.33 mL of 0.2454 M KOH solution to neutralize 35.0 mL of arsenic acid (H3AsO4),what is the concentration (M)of the arsenic acid solution?
(Short Answer)
4.8/5  (36)
(36)
Aqueous solutions of a compound did not form precipitates with Cl-,Br-,I-,SO42-,CO32-,PO43-,OH-,or S2-.This highly water-soluble compound produced the foul-smelling gas H2S when the solution was acidified.This compound is ________.
(Multiple Choice)
4.9/5  (35)
(35)
What volume (mL)of 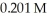 potassium hydroxide will it take to reach the equivalence point in a 16.3 mL aliquot of
potassium hydroxide will it take to reach the equivalence point in a 16.3 mL aliquot of 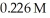 triprotic acid?
triprotic acid?
(Multiple Choice)
4.7/5  (28)
(28)
Aqueous potassium chloride will react with which one of the following in an exchange (metathesis)reaction?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 121 - 140 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)