Exam 16: Principles of Reactivity: Chemical Equilibria
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Given the following equilibria,
Ni2+(aq)+ 2 OH-(aq) 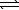 Ni(OH)2(s) K1 = 1.8 1015
Ni2+(aq)+ 4 CN-(aq)
Ni(OH)2(s) K1 = 1.8 1015
Ni2+(aq)+ 4 CN-(aq) 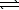 Ni(CN)42-(aq) K2 = 2.0 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq)
Ni(CN)42-(aq) K2 = 2.0 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq) 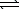 Ni(CN)42-(aq)+ 2 OH-(aq)
Ni(CN)42-(aq)+ 2 OH-(aq)
(Multiple Choice)
4.9/5  (43)
(43)
Given the equilibrium constants for the following reactions:
4Cu(s)+ O2(g)  2Cu2O(s),K1
4CuO(s)
2Cu2O(s),K1
4CuO(s)  2Cu2O(s)+ O2(g),K2
What is K for the system
2Cu(s)+ O2(g)
2Cu2O(s)+ O2(g),K2
What is K for the system
2Cu(s)+ O2(g)  2CuO(s)
Equivalent to?
2CuO(s)
Equivalent to?
(Multiple Choice)
4.7/5  (41)
(41)
The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows.
N2(g)+ 3 H2(g) 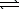 2 NH3(g) H = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
2 NH3(g) H = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
(Multiple Choice)
4.9/5  (35)
(35)
Sulfuryl chloride decomposes to sulfur dioxide and chlorine.
SO2Cl2(g) 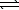 SO2(g)+ Cl2(g)
Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
SO2(g)+ Cl2(g)
Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
(Multiple Choice)
4.8/5  (35)
(35)
At a given temperature,K = 0.021 for the equilibrium:
PCl5(g)  PCl3(g)+ Cl2(g)
What is K for: Cl2(g)+ PCl3(g)
PCl3(g)+ Cl2(g)
What is K for: Cl2(g)+ PCl3(g)  PCl5(g)?
PCl5(g)?
(Multiple Choice)
4.9/5  (36)
(36)
For the reaction given below,2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container.
A(g)+ 2B(g)  C(g)
At equilibrium,the concentration of A is 0.223 mol/L.What is the value of Kc?
C(g)
At equilibrium,the concentration of A is 0.223 mol/L.What is the value of Kc?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements about the reaction quotient,Q,is false?
(Multiple Choice)
4.9/5  (38)
(38)
The equilibrium constant,Kc,for the decomposition of ammonium hydrogen sulfide is 1.8 10-4 at 25 C.NH4HS(s) 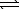 NH3(g)+ H2S(g)
If excess NH4HS(s)is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
NH3(g)+ H2S(g)
If excess NH4HS(s)is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
(Multiple Choice)
4.9/5  (31)
(31)
If the reaction quotient,Q,is greater than K in a gas phase reaction,then
(Multiple Choice)
4.9/5  (38)
(38)
Consider the reaction H2 + I2  2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
(Multiple Choice)
4.8/5  (38)
(38)
Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 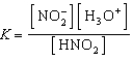
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following equilibria would not be affected by pressure changes at constant temperature?
(Multiple Choice)
4.7/5  (36)
(36)
If the reaction quotient,Q,is equal to K in a gas phase reaction,then
(Multiple Choice)
4.7/5  (46)
(46)
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
(Multiple Choice)
4.7/5  (31)
(31)
For the reaction TlSCN(s)  Tl+(aq)+ SCN-(aq),Kc =
Tl+(aq)+ SCN-(aq),Kc = 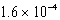 At 25 C.Which of the following concerning a 125 mL solution containing
At 25 C.Which of the following concerning a 125 mL solution containing 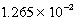 M Tl+,
M Tl+, 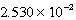 M SCN- and a large excess of TlSCN(s)is/are correct?
1)The mixture is at equilibrium.
2)Additional TlSCN(s)must precipitate to attain equilibrium.
3)The reaction quotient (Q)is greater than one.
M SCN- and a large excess of TlSCN(s)is/are correct?
1)The mixture is at equilibrium.
2)Additional TlSCN(s)must precipitate to attain equilibrium.
3)The reaction quotient (Q)is greater than one.
(Multiple Choice)
4.8/5  (48)
(48)
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?
(Multiple Choice)
4.9/5  (36)
(36)
Which expression correctly describes the equilibrium constant Kc for the following reaction?

(Multiple Choice)
4.9/5  (38)
(38)
A flask contains the following chemical system at equilibrium.
CuCO3(s) 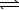 Cu2+(aq)+ 2 CO32-(aq)
Addition of which of the following substances will increase the solubility of CuCO3(s)in water?
1)aqueous hydrochloric acid
2)aqueous sodium carbonate
3)solid copper(II)carbonate
Cu2+(aq)+ 2 CO32-(aq)
Addition of which of the following substances will increase the solubility of CuCO3(s)in water?
1)aqueous hydrochloric acid
2)aqueous sodium carbonate
3)solid copper(II)carbonate
(Multiple Choice)
4.8/5  (27)
(27)
At a given temperature,0.0664 mol N2O4(g)is placed in a 1.00 L flask.After reaching equilibrium,the concentration of NO2(g)is 6.1 10-3 M.What is Kc for the reaction below?
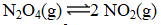
(Multiple Choice)
4.8/5  (42)
(42)
Consider the following equilibrium:
CO2(g)+ H2(g)  CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.038 mol CO2 and 0.022 mol H2 are placed in a 1.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.038 mol CO2 and 0.022 mol H2 are placed in a 1.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)