Exam 16: Principles of Reactivity: Chemical Equilibria
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g)  2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
(Multiple Choice)
4.9/5  (44)
(44)
Assume that the following chemical reaction is at equilibrium.
2 ICl(g) 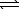 I2(g)+ Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
I2(g)+ Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
(Multiple Choice)
4.9/5  (39)
(39)
What is the reaction quotient,Q,for the equilibrium
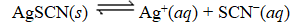 SCN-(aq)
When 0.4257 L of
SCN-(aq)
When 0.4257 L of 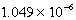 M Ag+ is combined with 0.2376 L of
M Ag+ is combined with 0.2376 L of 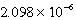 M SCN- in the presence of an excess of AgSCN(s)?
M SCN- in the presence of an excess of AgSCN(s)?
(Multiple Choice)
4.9/5  (32)
(32)
When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below.CO(g)+ 3H2(g) 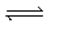 CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C?
CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C? 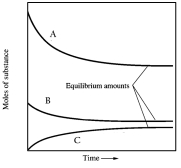
(Multiple Choice)
4.9/5  (52)
(52)
In an experiment,0.42 mol H2 and 0.42 mol I2 are mixed in a 1.00-L container,and the reaction forms HI.If Kc = 49.for this reaction,what is the equilibrium concentration of HI?
I2(g)+ H2(g)  2HI(g)
2HI(g)
(Multiple Choice)
4.7/5  (44)
(44)
At 25 C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below?
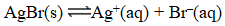
(Multiple Choice)
5.0/5  (43)
(43)
The reaction quotient,Q,for a system is 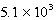 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 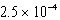 ,what will happen as the reaction mixture returns to equilibrium?
,what will happen as the reaction mixture returns to equilibrium?
(Multiple Choice)
4.8/5  (36)
(36)
For the reaction NO(g)+ ½ O2(g)  NO2(g)at 750°C,what is the relationship between Kc and Kp?
NO2(g)at 750°C,what is the relationship between Kc and Kp?
(Multiple Choice)
5.0/5  (40)
(40)
Which of the following is always true for a reaction where Kc is 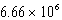 at 25 C?
at 25 C?
(Multiple Choice)
5.0/5  (31)
(31)
For which of the following reactions are the numerical values of Kp and Kc the same?
1. 2SO2(g) + O2(g)  2SO3(g)
2. N2(g) + O2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2NO(g)
3. H2(g)+ I2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2HI(g)
2SO3(g)
2. N2(g) + O2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2NO(g)
3. H2(g)+ I2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2HI(g)
(Multiple Choice)
4.8/5  (32)
(32)
For the equilibrium N2O4(g)  2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
(Multiple Choice)
4.8/5  (39)
(39)
Write the expression for K for the reaction of hydrofluoric acid with water.
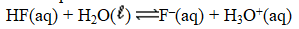
(Multiple Choice)
5.0/5  (37)
(37)
Assume that the following chemical reaction is at equilibrium.
C(s)+ H2O(g) 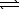 H2(g)+ CO(g)
Which of the following statements is/are CORRECT?
1)Increasing the concentration of H2(g)will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g).
2)Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
3)Increasing the amount of C(s)will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
H2(g)+ CO(g)
Which of the following statements is/are CORRECT?
1)Increasing the concentration of H2(g)will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g).
2)Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
3)Increasing the amount of C(s)will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
(Multiple Choice)
4.9/5  (36)
(36)
A mixture of nitrogen and hydrogen was allowed to come to equilibrium at a given temperature.
3H2 + N2  2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2,3.2 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2,3.2 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements is/are CORRECT?
1)For a chemical system,if the reaction quotient (Q)is greater than K,reactant must be converted to products to reach equilibrium.
2)For a chemical system at equilibrium,the forward and reverse rates of reaction are equal.
3)For a chemical system at equilibrium,the concentrations of products divided by the concentrations of reactants equals one.
(Multiple Choice)
4.9/5  (42)
(42)
Nitrosyl bromide decomposes according to the chemical equation below.2 NOBr(g) 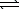 2 NO(g)+ Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
2 NO(g)+ Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following statements is/are CORRECT?
1)Product concentrations appear in the numerator of an equilibrium constant expression.
2)A reaction favors the formation of products if K >> 1.
3)Stoichiometric coefficients are used as exponents in an equilibrium constant expression.
(Multiple Choice)
4.8/5  (40)
(40)
Consider the reaction
A(aq) 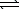 2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?
2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)