Exam 2: The Components of Matter
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The compound, (NH4)2S,can be used in analysis for trace amounts of metals present in a sample.What is its name?
(Multiple Choice)
4.8/5  (32)
(32)
For each of the following names,write down the corresponding formula,including charge where appropriate (atomic numbers and mass numbers are not required):
a.zinc ion
b.nitrite ion
c.carbonic acid
d.cyanide ion
(Essay)
4.8/5  (28)
(28)
Barium sulfate is used in manufacturing photographic paper.What is its formula?
(Multiple Choice)
4.9/5  (34)
(34)
Iodine pentafluoride reacts slowly with glass and violently with water.Determine its molecular mass.
(Multiple Choice)
4.8/5  (34)
(34)
Who is credited with first measuring the charge of the electron?
(Multiple Choice)
4.9/5  (31)
(31)
Which one of the following statements about atoms and subatomic particles is correct?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following combinations of names and formulas of ions is incorrect?
(Multiple Choice)
4.8/5  (33)
(33)
Rutherford bombarded gold foil with alpha ( )particles and found that a small percentage of the particles were deflected.Which of the following was not accounted for by the model he proposed for the structure of atoms?
(Multiple Choice)
4.7/5  (33)
(33)
a.Give the names of the following ions:
(i)O22-
(ii)SO42-
b.Write down the formulas of the following ions:
(i)ammonium
(ii)nitrate
(Essay)
4.9/5  (35)
(35)
Zinc acetate is used in preserving wood and in manufacturing glazes for porcelain.What is its formula?
(Multiple Choice)
4.7/5  (30)
(30)
Who is credited with measuring the mass/charge ratio of the electron?
(Multiple Choice)
4.8/5  (34)
(34)
The substance,CaSe,is used in materials which are electron emitters.What is its name?
(Multiple Choice)
4.9/5  (39)
(39)
Ammonium sulfate, (NH4)2SO4,is a fertilizer widely used as a source of nitrogen.Calculate its molecular mass.
(Multiple Choice)
4.8/5  (40)
(40)
Bromine is the only nonmetal that is a liquid at room temperature.Consider the isotope bromine-81, 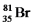 .Select the combination which lists the correct atomic number,neutron number,and mass number,respectively.
.Select the combination which lists the correct atomic number,neutron number,and mass number,respectively.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 61 - 80 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)