Exam 2: The Components of Matter
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Iron (III)chloride hexahydrate is used as a coagulant for sewage and industrial wastes.What is its formula?
(Multiple Choice)
4.7/5  (29)
(29)
For each of the following elements,indicate whether it is a metal,a non-metal or a metalloid:
a.S
b.Ge
c.Hg
d.H
e.I
f.Si
(Essay)
4.8/5  (34)
(34)
Modern studies have shown that the Law of Multiple Proportions is not valid.
(True/False)
4.8/5  (27)
(27)
Silicon,which makes up about 25% of Earth's crust by mass,is used widely in the modern electronics industry.It has three naturally occurring isotopes,28Si,29Si,and 30Si.Calculate the atomic mass of silicon.
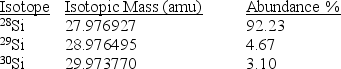
(Multiple Choice)
4.9/5  (32)
(32)
Kaolinite,a clay mineral with the formula Al4Si4O10(OH)8,is used as a filler in slick-paper for magazines and as a raw material for ceramics.Analysis shows that 14.35 g of kaolinite contains 8.009 g of oxygen.Calculate the mass percent of oxygen in kaolinite.
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following combinations of names and formulas of ions is incorrect?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following combinations of names and formulas of ions is incorrect?
(Multiple Choice)
4.8/5  (31)
(31)
The molecular formula of a compound provides more information than its structural formula.
(True/False)
4.8/5  (30)
(30)
State the two important experimental results (and the names of the responsible scientists)which enabled the mass of the electron to be determined.
(Essay)
4.8/5  (30)
(30)
A red glaze on porcelain can be produced by using MnSO4.What is its name?
(Multiple Choice)
4.8/5  (31)
(31)
Fill in the blank spaces and write out all the symbols in the left hand column in full,in the form 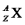 (i.e. ,include the appropriate values of Z and A as well as the correct symbol X).
(i.e. ,include the appropriate values of Z and A as well as the correct symbol X).

(Essay)
4.9/5  (41)
(41)
Sodium chromate is used to protect iron from corrosion and rusting.Determine its molecular mass.
(Multiple Choice)
4.7/5  (40)
(40)
The substance,CoCl2,is useful as a humidity indicator because it changes from pale blue to pink as it gains water from moist air.What is its name?
(Multiple Choice)
4.8/5  (32)
(32)
Lithium forms compounds which are used in dry cells and storage batteries and in high-temperature lubricants.It has two naturally occurring isotopes,6Li (isotopic mass = 6.015121 amu)and 7Li (isotopic mass = 7.016003 amu).Lithium has an atomic mass of 6.9409 amu.What is the percent abundance of lithium-6?
(Multiple Choice)
4.8/5  (27)
(27)
Calculate the molecular masses of the following:
a.Cl2
b.H2O2
c.(NH4)2SO4
d.Ba(NO3)2
(Essay)
4.8/5  (36)
(36)
Showing 21 - 40 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)