Exam 13: The Properties of Solutions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Procaine hydrochloride ( 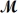 = 272.77 g/mol)is used as a local anesthetic.Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
= 272.77 g/mol)is used as a local anesthetic.Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
(Multiple Choice)
4.8/5  (33)
(33)
A 0.100 m MgSO4 solution has a freezing point of -0.23°C.What is the van't Hoff factor for this solution?
Kf = 1.86°C/m
(Multiple Choice)
4.8/5  (33)
(33)
Children under the age of six with more than 0.10 ppm of lead in their blood can suffer a reduction in I.Q.or have behavior problems.What is the molality of a solution which contains 0.10 ppm of lead?
(Multiple Choice)
4.7/5  (39)
(39)
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers.Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution.
(Multiple Choice)
4.7/5  (41)
(41)
Benzaldehyde ( 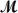 = 106.1 g/mol),also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m,freezing point of pure ethanol = -117.3°C.
= 106.1 g/mol),also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m,freezing point of pure ethanol = -117.3°C.
(Multiple Choice)
4.8/5  (35)
(35)
Diethyl ether has a vapor pressure of 400.0 torr at 18°C.When a sample of benzoic acid is dissolved in ether,the vapor pressure of the solution is 342 torr.What is the mole fraction of benzoic acid in the solution?
(Multiple Choice)
4.9/5  (33)
(33)
Predict the van't Hoff factor (i)for each of the following solutes:
a.CaCl2
b.Fe2(SO4)3
c.NH4Cl
(Essay)
4.8/5  (43)
(43)
Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 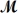 = 86.18 g/mol)at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol)at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
(Multiple Choice)
4.7/5  (46)
(46)
Aqueous ammonia is commercially available in a solution that is 28% (w/w)ammonia.What is the mole fraction of ammonia in such a solution?
(Multiple Choice)
4.7/5  (34)
(34)
Octane and nonane are liquids which are components of gasoline.Their vapor pressures at 25°C are 13.9 torr and 4.7 torr,respectively.What is the vapor pressure of a mixture consisting of 1 mole of each of these compounds?
(Short Answer)
4.9/5  (33)
(33)
From the following list of aqueous solutions and water,select the one with the highest boiling point.
(Multiple Choice)
4.7/5  (41)
(41)
Copper(II)bromide is used as a wood preservative.What mass of CuBr2 is needed to prepare 750.0 mL of a 1.25 M solution?
(Multiple Choice)
4.9/5  (45)
(45)
Isoamyl salicylate ( 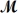 = 208.25 g/mol)has a pleasant aroma and is used in perfumes and soaps.Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
= 208.25 g/mol)has a pleasant aroma and is used in perfumes and soaps.Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
(Multiple Choice)
4.7/5  (41)
(41)
How many moles of bromide ions are present in 750.0 mL of 1.35 M MgBr2?
(Multiple Choice)
4.8/5  (34)
(34)
Lysine is an amino acid that is an essential part of nutrition but which is not synthesized by the human body.What is the molar mass of lysine if 750.0 mL of a solution containing 8.60 g of lysine has an osmotic pressure of 1.918 atm?
T = 25.0°C
(Multiple Choice)
4.9/5  (37)
(37)
How many moles of sulfate ions are present in 1.0 L of 0.5 M Li2SO4?
(Multiple Choice)
4.8/5  (42)
(42)
A 0.100 m K2SO4 solution has a freezing point of -0.43°C.What is the van't Hoff factor for this solution?
Kf = 1.86°C/m
(Multiple Choice)
4.8/5  (34)
(34)
Cadmium bromide is used in photography and lithography.Calculate the molality of a solution prepared by dissolving 45.38 g of CdBr2 in 375.0 g of water.
(Multiple Choice)
4.8/5  (28)
(28)
A 2.0% (w/v)solution of sodium hydrogen citrate,Na2C6H6O7,which also contains 2.5% (w/v)of dextrose,C6H12O6,is used as an anticoagulant for blood which is to be used for transfusions.What is the molarity of the sodium hydrogen citrate in the solution?
(Multiple Choice)
4.8/5  (40)
(40)
Carbon tetrachloride,once widely used in fire extinguishers and as a dry cleaning fluid,has been found to cause liver damage to those exposed to its vapors over long periods of time.What is the boiling point of a solution prepared by dissolving 375 g of sulfur (S8, 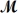 = 256.5g/mol)in 1250 g of CCl4? Kb = 5.05°C/m,boiling point of pure CCl4 = 76.7°C?
= 256.5g/mol)in 1250 g of CCl4? Kb = 5.05°C/m,boiling point of pure CCl4 = 76.7°C?
(Multiple Choice)
4.9/5  (47)
(47)
Showing 41 - 60 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)