Exam 13: The Properties of Solutions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Barbiturates are synthetic drugs used as sedatives and hypnotics.Barbital ( 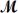 = 184.2 g/mol)is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid?
Kb = 3.07°C/m,boiling point of pure acetic acid = 117.9°C
= 184.2 g/mol)is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid?
Kb = 3.07°C/m,boiling point of pure acetic acid = 117.9°C
(Multiple Choice)
4.8/5  (35)
(35)
The concentration of iodine in sea water is 60.parts per billion by mass.If one assumes that the iodine exists in the form of iodide anions,what is the molarity of iodide in sea water? (The density of sea water is 1.025 g/mL. )
(Multiple Choice)
4.8/5  (35)
(35)
A 0.137 m solution of sodium ferrocyanide (NaFe(CN)6.10H2O)in water has a freezing point depression of 0.755°C.Calculate the van't Hoff factor (i)under these conditions.(Kf = 1.86°C/m)
(Short Answer)
4.7/5  (38)
(38)
A solution of sucrose (sugar)in water is in equilibrium with solid sucrose.If more solid sucrose is now added,with stirring,
(Multiple Choice)
4.7/5  (40)
(40)
Hexachlorophene is used as a disinfectant in germicidal soaps.What mass of hexachlorophene ( 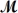 = 406.9 g/mol)must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C?
Kb = 3.63°C/m,boiling point of pure chloroform = 61.70°C
= 406.9 g/mol)must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C?
Kb = 3.63°C/m,boiling point of pure chloroform = 61.70°C
(Multiple Choice)
4.9/5  (37)
(37)
The Henry's Law constant (k)for carbon monoxide in water at 25°C is 9.71 10-4 mol/(L·atm).How many grams of CO will dissolve in 1.00 L of water if the partial pressure of CO is 2.75 atm?
(Multiple Choice)
4.8/5  (39)
(39)
What volume of concentrated (14.7 M)phosphoric acid is needed to prepare 25.0 L of 3.0 M H3PO4?
(Multiple Choice)
4.9/5  (32)
(32)
Determine the freezing point of a solution which contains 0.31 mol of sucrose in 175 g of water.Kf = 1.86°C/m
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following aqueous solutions should demonstrate the most non-ideal behavior?
(Multiple Choice)
4.9/5  (45)
(45)
In general,water is a good solvent for both polar and non-polar compounds.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 73 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)